Actividad bioestimulante de ácidos húmicos derivados de vermicompost de estiércol caprino y lignito en relación con su estructura e interacción con una cepa PGPR en condiciones semiáridas
Resumen
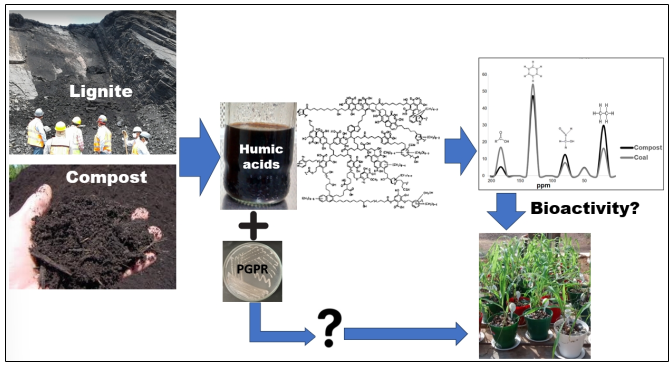
El uso de bioestimulantes vegetales a base materia orgánica humificada (MOH) y PGPR’s es una tecnología en vía de consolidación para promover la productividad agrícola bajo condiciones marginales. Sin embargo, la bioactividad de los estimulantes húmicos varía de acuerdo con su naturaleza química y aun es necesario explicar los efectos sobre la aplicación conjunta de estos dos agentes bioestimulantes. En este trabajo se analizó la relación entre la estructura y bioactividad de ácidos húmicos obtenidos de lombricompost de estiércol caprino (HAVC) y de un carbón tipo lignito (HAC), así como el efecto promotor del crecimiento vegetal de cada uno de estos ácidos húmicos (AH) aplicados en conjunto de la cepa Bacillus mycoides BSC25 (Bm), sobre plantas de maíz, bajo condiciones de aridez. Para ello se determinó la composición supramolecular de los HA, su bioactividad fue evaluada mediante el test de elongación de coleóptilos de maíz, se desarrollaron ensayos de bioestimulación sobre plántulas de maíz en cámara de crecimiento, finalmente se desarrolló un ensayo de bioestimulación en maíz en condiciones de campo en una zona semiárida. Los HAC causaron elongación de coleóptilos a una concentración inferior (25-50 mg CL), en comparación con los HAVC, que causaron el mismo efecto a una concentración más alta (100-200 mg CL); se encontró una asociación entre estos resultados y la composición supramolecular de los HA. La bioactividad de los HAC estuvo asociada a su contenido de oxígeno, grupos aromáticos y carboxílicos; mientras que la bioactividad de los HAVC se correlacionó con su contenido de carbohidratos, carbono alifático y de hidrógeno. La aplicación de ambos HA, Bm y la aplicación conjunta HA- Bm, promovió la producción de biomasa foliar de maíz en cámara de crecimiento y en condiciones de campo. Esto se puede atribuir a los efectos hormone-like de los HA y a la actividad PGPR de Bm. Aunque los HA fueron aplicados por aspersión foliar, mostraron bioactividad a nivel radicular. Los resultados de biomasa radicular en campo indican un efecto PGPR de Bm, el cual no se vio modificado por la aplicación conjunta con HAVC. Sin embargo, la producción de mazorcas en condiciones de campo fue promovida por la actividad de Bm, pero este efecto fue revertido por la aplicación conjunta Bm-HAC y Bm-HAVC. Estos resultados están asociados a los efectos hormonales de los HA y de posibles efectos aditivos tras la inoculación con Bm.
Palabras clave
Compuestos bioactivos, Zea mays, Bacillus mycoides, Manejo de tierra secas, Inseguridad alimentaria, Biotecnología
Citas
- Aguiar, N.O., E.H. Novotny, A.L. Oliveira, V.M. Rumjanek, F.L. Olivares, and L.P. Canellas. 2013. Prediction of humic acids bioactivity using spectroscopy and multivariate analysis. J. Geochem. Explor. 129, 95-102. Doi: https://doi.org/10.1016/j.gexplo.2012.10.005
- Aguiar, N.O., F.L. Olivares, E.H. Novotny, and L.P. Canellas. 2018. Changes in metabolic profiling of sugarcane leaves induced by endophytic diazotrophic bacteria and humic acids. PeerJ 6, e5445. Doi: https://doi.org/10.7717/peerj.5445
- Ahmad, P., S. Rasool, A. Gul, S.A. Sheikh, N.A. Akram, M. Ashraf, A.M. Kazi, and S. Gucel. 2016. Jasmonates: multifunctional roles in stress tolerance. Front. Plant Sci. 7, 813. Doi: https://doi.org/10.3389/fpls.2016.00813
- Ait Baddi, G., M. Hafidi, V. Gilard, and J.-C. Revel. 2003. Characterization of humic acids produced during composting of olive mill wastes: elemental and spectroscopic analyses (FTIR and 13C-NMR). Agronomie 23(7), 661-666. Doi: https://doi.org/10.1051/agro:2003042
- Araújo, K.V., M. Pittarello, P. Carletti, A.R.M. Campos, and L.B. Dobbss. 2021. Structural characterization and bioactivity of humic and fulvic acids extracted from preserved and degraded Brazilian Cerrado Biomes Soils. Eurasian Soil Sci. 54(1), 16-25. Doi: https://doi.org/10.1134/S1064229322030024
- Bacilio, M., M. Moreno, D.R. Lopez-Aguilar, and Y. Bashan, 2017. Scaling from the growth chamber to the greenhouse to the field: demonstration of diminishing effects of mitigation of salinity in peppers inoculated with plant growth-promoting bacterium and humic acids. Appl. Soil Ecol. 119, 327-338. Doi: https://doi.org/10.1016/j.apsoil.2017.07.002
- Bashan, Y., B.G. Salazar, M. Moreno, B.R. Lopez, and R.G. Linderman. 2012. Restoration of eroded soil in the Sonoran Desert with native leguminous trees using plant growth-promoting microorganisms and limited amounts of compost and water. J. Environ. Manage. 102, 26-36. Doi: https://doi.org/10.1016/j.jenvman.2011.12.032
- Beleño-Carrillo, J., L. Gómez-Gómez, and N.O. Valero-Valero. 2022. Bacillus mycoides y ácidos húmicos como bioestimulantes de fríjol caupí bajo estrés por salinidad. Rev. U.D.C.A Act. Div. Cient. 25(2), e1974. Doi: https://doi.org/10.31910/rudca.v25.n2.2022.1974
- Blouin, M. 2018. Chemical communication: An evidence for co-evolution between plants and soil organisms. Appl. Soil Ecol. 123, 409-415. Doi: https://doi.org/10.1016/j.apsoil.2017.10.028
- Bratkova, S., K. Nikolova, P. Genova, and A. Angelov. 2021. Use of plant growth-promoting bacteria and humic acids for phytostabilization of acid-generating mining wastes. Res Sq. Preprint v1. Doi: https://doi.org/10.21203/rs.3.rs-550365/v1
- Brito-Campo, H.L., M.F. Ayala-Santamaría, K.J. Barros-Escalante, J.G. Cubillos-Hinojosa, M.F. Pantoja-Guerra, N.O. Valero, and L. Gómez. 2022. PGPR activity of coal solubilizing bacteria. Rev. Fac. Agron.(LUZ) 39(2), e223932. Doi: https://doi.org//10.47280/RevFacAgron(LUZ).v39.n2.10
- Bulgari, R., G. Franzoni, and A. Ferrante. 2019. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 9(6), 306. Doi: https://doi.org/10.3390/agronomy9060306
- Canellas, L.P., N.O.A. Canellas, L.E.S.S. Irineu, F.L. Olivares, and A. Piccolo. 2020. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 7(1), 12. Doi: https://doi.org/10.1186/s40538-020-00178-4
- Canellas, L.P., D.J. Dantas, N.O. Aguiar, L.E.P. Peres, A. Zsögön, F.L. Olivares, L.B. Dobbss, A.R. Façanha, A. Nebibioso, and A. Piccolo. 2011. Probing the hormonal activity of fractionated molecular humic components in tomato auxin mutants. Ann. Appl. Biol. 159(2), 202-211. Doi: https://doi.org/10.1111/j.1744-7348.2011.00487.x
- Canellas, L.P., A. Piccolo, L.B. Dobbss, R. Spaccini, F.L. Olivares, D.B. Zandonadi, and A.R. Façanha. 2010. Chemical composition and bioactivity properties of size-fractions separated from a vermicompost humic acid. Chemosphere 78(4), 457-466. Doi: https://doi.org/10.1016/j.chemosphere.2009.10.018
- Canellas, L.P. and F.L. Olivares. 2014. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 1(1), 3. Doi: https://doi.org/10.1186/2196-5641-1-3
- Canellas, L.P., F.L. Olivares, N.O.A. Canellas, P. Mazzei, and A. Piccolo. 2019. Humic acids increase the maize seedlings exudation yield. Chem. Biol. Technol. Agric. 6(1), 3. Doi: https://doi.org/10.1186/s40538-018-0139-7
- Canellas, L.P., F.L. Olivares, A.L. Okorokova-Façanha, and A.R. Façanha. 2002. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol. 130(4), 1951-1957. Doi: https://doi.org/10.1104/pp.007088
- Cavagnaro, T.R. 2014. Impacts of compost application on the formation and functioning of arbuscular mycorrhizas. Soil Biol. Biochem. 78, 38-44. Doi: https://doi.org/10.1016/j.soilbio.2014.07.007
- Chang, R.R., R. Mylotte, M.H.B. Hayes, R. Mclnerney, and Y.M. Tzou. 2014. A comparison of the compositional differences between humic fractions isolated by the IHSS and exhaustive extraction procedures. Naturwissenschaften 101(3), 197-209. Doi: https://doi.org/10.1007/s00114-013-1140-4
- Conklin, P.A., J. Strable, S. Li, and M.J. Scanlon. 2019. On the mechanisms of development in monocot and eudicot leaves. New Phytol. 221(2), 706-724. Doi: https://doi.org/10.1111/nph.15371
- Da Piedade Melo, A., F.L. Olivares, L.O. Médici, A. Torres-Neto, L.B. Dobbss, and L.P. Canellas. 2017. Mixed rhizobia and Herbaspirillum seropedicae inoculations with humic acid-like substances improve water-stress recovery in common beans. Chem. Biol. Technol. Agric. 4(1), 6. Doi: https://doi.org/10.1186/s40538-017-0090-z
- De Sanfilippo, E.C., J.A. Argüello, G. Abdala, and G.A. Orioli. 1990. Content of auxin-inhibitor-and gibberellin-like substances in humic acids. Biol. Plant. 32(5), 346-351. Doi: https://doi.org/10.1007/BF02898497
- Du Jardin, P. 2015. Plant biostimulants: definition, concept, main categories and regulation. Sci. Hortic. 196, 3-14. Doi: https://doi.org/10.1016/j.scienta.2015.09.021
- Elmongy, M.S., X. Wang, H. Zhou, and Y. Xia. 2020. Humic acid and auxins induced metabolic changes and differential gene expression during adventitious root development in azalea microshoots. HortScience 55(6), 926-935. Doi: https://doi.org/10.21273/HORTSCI14885-20
- FAO. 2022. Agricultura de Conservación. In: https://www.fao.org/3/cb8350es/cb8350es.pdf; consulted: February, 2023.
- Fischer, T. 2017. Humic supramolecular structures have polar surfaces and unpolar cores in native soil. Chemosphere 183, 437-443. Doi: https://doi.org/10.1016/j.chemosphere.2017.05.125
- García A.C., L.G. Ambrosio de Souza, M.G. Pereira, R.N. Castro, J.M. García-Mina, E. Zonta, F.J.G. Lisboa, and R.L.L. Berbara. 2016a. Structure-property-function relationship in humic substances to explain the biological activity in plants. Sci. Rep. 6(1), 20798. Doi: https://doi.org/10.1038/srep20798
- García, A.C., L. Azevedo, F. Guridi, V.M. Rumjanek, R.N. Castro, F.S. Santos, L.G.A. Souza, and R.L.L. Berbara. 2014. Potentialities of vermicompost humic acids to alleviate water stress in rice plants (Oryza sativa L.). J. Geochem. Explor. 136, 48-54. Doi: https://doi.org/10.1016/j.gexplo.2013.10.005
- García, A.C., L. Azevedo, L.G.A. Souza, O.C. Huertas, A. Zonta, E.T. Martins, J.M. García-Mina, and R.L.L. Berbara. 2016b. Vermicompost humic acids modulate the accumulation and metabolism of ROS in rice plants. J. Plant Physiol. 192, 56-63. Doi: https://doi.org/10.1016/j.jplph.2016.01.008
- García, A.C., O.C. Huertas, D. Martínez, V.S. Almeida, L.P. Canellas, J.M. García-Mina, and R.L.L. Berbara. 2018. Structure-function relationship of vermicompost humic fractions for use in agriculture. J. Soils Sediments 18(4), 1365-1375. Doi: https://doi.org/10.1007/s11368-016-1521-3
- García, A.C., T.A. Van Tol de Castro, L.A. Santos, O.C.H. Tavares, R.N. Castro, R.C.L. Berbara, and J.M. García-Mina. 2019. Structure-property-function relationship of humic substances in modulating the root growth of plants: a review. J. Environ. Qual. 48(6), 1622-1632. Doi: https://doi.org/10.2134/jeq2019.01.0027
- Giannouli, A., S. Kalaitzidis, G. Siavalas, A. Chatziapostolou, K. Christanis, S. Papazisimou, C. Papanicolou, and A. Foscolos. 2009. Evaluation of greek low-rank coals as potential raw material for the production of soil amendments and organic fertilizers. Int. J. Coal Geol. 77(3-4), 383-393. Doi: https://doi.org/10.1016/j.coal.2008.07.008
- Gualdrón, R. 2011. Cerrejón: hacia la rehabilitación de las tierras intervenidas por la minería a cielo abierto. El Cerrejón, Bogota.
- Jindo, K., F.L. Olivares, D.J.P. Malcher, M.A. Sánchez-Monedero, C. Kempenaar, and L.P. Canellas. 2020. From lab to field: role of humic substances under open-field and greenhouse conditions as biostimulant and biocontrol agent. Front. Plant Sci. 11, 426. Doi: https://doi.org/10.3389/fpls.2020.00426
- Li, J., J. Wang, H. Liu, C.A. Macdonald, and B.K. Singh. 2022. Application of microbial inoculants significantly enhances crop productivity: a meta-analysis of studies from 2010 to 2020. J. Sustain. Agric. Environ. 1(3), 216-225. Doi: 10.1002/sae2.12028
- McSteen, P. 2010. Auxin and monocot development. Cold Spring Harb. Perspect. Biol. 2010(2), a001479. Doi: https://doi.org/10.1101/cshperspect.a001479
- Mora, V., E. Bacaicoa, R. Baigorri, A.M. Zamarreño, and J.M. García-Mina. 2014. NO and IAA key regulators in the shoot growth promoting action of humic acid in Cucumis sativus L. J. Plant Growth Regul. 33(2), 430-439. Doi: https://doi.org/10.1007/s00344-013-9394-9
- Muscolo, A., F. Bovalo, F. Gionfriddo, and S. Nardi. 1999. Earthworm humic matter produces auxin-like effects on Daucus carota cell growth and nitrate metabolism. Soil Biol. Biochem. 31(9), 1303-1311. Doi: https://doi.org/10.1016/S0038-0717(99)00049-8
- Muscolo, A., D. Pizzeghello, O. Francioso, S. Sanchez, and S. Nardi. 2020. Effectiveness of humic substances and phenolic compounds in regulating plant-biological functionality. Agronomy 10(10), 1553. Doi: https://doi.org/10.3390/agronomy10101553
- Muscolo, A., M. Sidari, E. Attinà, O. Francioso, V. Tugnoli, and S. Nardi. 2007a. Biological activity of humic substances is related to their chemical structure. Soil Sci. Soc. Am. J. 71(1), 75-85. Doi: https://doi.org/10.2136/sssaj2006.0055
- Muscolo, A., M. Sidari, O. Francioso, V. Tugnoli, and S. Nardi. 2007b. The auxin-like activity of humic substances is related to membrane interactions in carrot cell cultures. J. Chem. Ecol. 33(1), 115-129. Doi: https://doi.org/10.1007/s10886-006-9206-9
- Muscolo, A., M. Sidari, and S. Nardi. 2013. Humic substance: relationship between structure and activity. Deeper information suggests univocal findings. J. Geochem. Explor. 129, 57-63. Doi: https://doi.org/10.1016/j.gexplo.2012.10.012
- Nardi, S., D. Pizzeghello, and A. Ertani. 2018. Hormone-like activity of the soil organic matter. Appl. Soil Ecol. 123, 517-520. Doi: https://doi.org/10.1016/j.apsoil.2017.04.020
- Nardi, S., D. Pizzeghello, A. Muscolo, and A. Vianello. 2002. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 34(11), 1527-1536. Doi: https://doi.org/10.1016/S0038-0717(02)00174-8
- Nardi, S., D. Pizzeghello, M. Schiavon, and A. Ertani. 2016. Plant biostimulants: physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 73(1), 18-23. Doi: https://doi.org/10.1590/0103-9016-2015-0006
- Nardi, S., M. Schiavon, and O. Francioso. 2021. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 26(8), 2256. Doi: https://doi.org/10.3390/molecules26082256
- Nebbioso, A. and A. Piccolo. 2011. Basis of a humeomics science: chemical fractionation and molecular characterization of humic biosuprastructures. Biomacromolecules 12(4), 1187-1199. Doi: https://doi.org/10.1021/bm101488e
- Nelissen, H., N. Gonzalez, and D. Inzé. 2016. Leaf growth in dicots and monocots: so different yet so alike. Curr. Opin. Plant Biol. 33, 72-76. Doi: https://doi.org/10.1016/j.pbi.2016.06.009
- Nunes, R.O., G.A. Domociano, W.S. Alves, A.C.A. Melo, F.C.S. Nogueira, L.P. Canellas, F.L. Olivares, R.B. Zingali, and M.R. Soares. 2019. Evaluation of the effects of humic acids on maize root architecture by label-free proteomics analysis. Sci. Rep. 9(1), 12019. Doi: https://doi.org/10.1038/s41598-019-48509-2
- Ojwang’ L.M. and R.L. Cook. 2013. Environmental conditions that influence the ability of humic acids to induce permeability in model biomembranes. Environ. Sci. Technol. 47(15), 8280-8287. Doi: https://doi.org/10.1021/es4004922
- Olaetxea, M., V. Mora, E. Bacaicoa, R. Baigorri, M. Garnica, M. Fuentes, A.M. Zamarreño, L. Spíchal, and J.M. GArcía-Mina. 2019. Root ABA and H+‐ATPase are key players in the root and shoot growth‐promoting action of humic acids. Plant Direct 3(10), e00175. Doi: https://doi.org/10.1002/pld3.175
- Pantoja-Guerra, M. and N. Valero-Valero. 2020. Design of a bio-conditioner prototype for the treatment of degraded soils: biomass production and lignite formulation for Microbacterium sp. CSB3. Chem. Biol. Technol. Agric. 7, 3. Doi: https://doi.org/10.1186/s40538-019-0167-y
- Pantoja-Guerra, M., N. Valero-Valero, and C.A. Ramírez. 2023. Total auxin level in the soil–plant system as a modulating factor for the effectiveness of PGPR inocula: a review. Chem. Biol. Technol. Agric. 10, 6. Doi: https://doi.org/10.1186/s40538-022-00370-8
- Pizzeghello, D., O. Francioso, A. Ertani, A. Muscolo, and S. Nardi. 2013. Isopentenyladenosine and cytokinin-like activity of different humic substances. J. Geochem. Explor. 129, 70-75. Doi: https://doi.org/10.1016/j.gexplo.2012.10.007
- Pizzeghello, D., M. Schiavon, O. Francioso, F.D. Vecchia, A. Ertani, and S. Nardi. 2020. Bioactivity of size-fractionated and unfractionated humic substances from two forest soils and comparative effects on N and S metabolism, nutrition, and root anatomy of Allium sativum L. Front. Plant Sci. 11, 1203. Doi: https://doi.org/10.3389/fpls.2020.01203
- Reeves, T.G., G. Thomas, and G. Ramsay. 2016. Save and grow in practice: maize, rice, wheat. A guide to sustainable cereal production. FAO, Rome.
- Ritchie, J.D. and E.M. Perdue. 2008. Analytical constraints on acidic functional groups in humic substances. Org. Geochem. 39(6), 783-799. Doi: https://doi.org/10.1016/j.orggeochem.2008.03.003
- Rojas, D., M. Contreras, and G. Santoyo. 2013. Mecanismos de estimulación del crecimiento vegetal en bacterias del género Bacillus. Biológicas 15(2), 36-41.
- Roomi, S., A. Masi, G.B. Conselvan, S. Trevisan, S. Quaggiotti, M. Pivato, G. Arrigoni, T. Yasmin, and P. Carletti. 2018. Protein profiling of Arabidopsis roots treated with humic substances: insights into the metabolic and interactome networks. Front. Plant Sci. 9, 1812. Doi: https://doi.org/10.3389/fpls.2018.01812
- Scaglia, B., R.R. Nunes, M.O.O. Rezende, F. Tambone, and F. Adani. 2016. Investigating organic molecules responsible of auxin-like activity of humic acid fraction extracted from vermicompost. Sci. Total Environ. 562, 289-295. Doi: https://doi.org/10.1016/j.scitotenv.2016.03.212
- Shah, Z.H., H.F. Rehman, T. Akhtar, H. Alsamadany, B.T. Hamooh, T. Mujtaba, I. Daur, Y.A. Zahrani, H.A.S. Alzahrani, S. Ali, S.H. Yang, and G. Chung. 2018. Humic substances: determining potential molecular regulatory processes in plants. Front. Plant Sci. 9, 263. Doi: https://doi.org/10.3389/fpls.2018.00263
- Sharma, H.S.S., C. Selby, E. Carmichael, C. McRoberts, J.R. Rao, P. Ambriosio, M. Chiurazzi, M. Pucci, and T. Martin. 2016. Physicochemical analyses of plant biostimulant formulations and characterisation of commercial products by instrumental techniques. Chem. Biol. Technol. Agric. 3, 13. Doi: https://doi.org/10.1186/s40538-016-0064-6
- Spaccini, R. and A. Piccolo. 2009. Molecular characteristics of humic acids extracted from compost at increasing maturity stages. Soil Biol. Biochem. 41(6), 1164-1172. Doi: https://doi.org/10.1016/j.soilbio.2009.02.026
- Tahiri, A., A. Richel, J. Destain, P. Druart, P. Thonart, and M. Ongena. 2016. Comprehensive comparison of the chemical and structural characterization of landfill leachate and leonardite humic fractions. Anal. Bioanal. Chem. 408(7), 1917-1928. Doi: https://doi.org/10.1007/s00216-016-9305-6
- Tejera-Hernández, B., M.M. Rojas-Badia, and M. Heydrich-Pérez. 2011. Potencialidades del género Bacillus en la promoción del crecimiento vegetal y el control biológico de hongos fitopatógenos. Rev. CENIC Cienc. Biol. 42(3), 131-138.
- Trevisan, S., O. Francioso, S. Quaggiotti, and S. Nardi. 2010. Humic substances biological activity at the plant-soil interface. Plant Signal. Behav. 5(6), 635-643. Doi: https://doi.org/10.4161/psb.5.6.11211
- Valero, N., L. Gómez, M. Pantoja, and R. Ramírez. 2014. Production of humic substances through coal-solubilizing bacteria. Brazilian J. Microbiol. 45(3), 911-918. Doi: https://doi.org/10.1590/s1517-83822014000300021
- Valero, N.O., L.C. Gómez, and L.M. Melgarejo. 2018b. Supramolecular characterization of humic acids obtained through the bacterial transformation of a low rank coal. J. Braz. Chem. Soc. 29(9), 1842-1853. Doi: https://doi.org/10.21577/0103-5053.20180060
- Valero, N., L.M. Melgarejo, and R. Ramírez. 2016. Effect of low-rank coal inoculated with coal solubilizing bacteria on edaphic materials used in post-coal-mining land reclamation: a greenhouse trial. Chem. Biol. Technol. Agric. 3, 20. Doi: https://doi.org/10.1186/s40538-016-0068-2
- Valero, N., J.A. Salgado, and D. Corzo. 2018a. Metodología sencilla para evaluar bioactividad de ácidos húmicos obtenidos de lignito mediante extracción alcalina y bacterias solubilizadoras de carbón. Inf. Tecnol. 29(4), 75-82. Doi: https://doi.org/10.4067/S0718-07642018000400075
- Valero-Valero, N.-O., C.M. Vergel-Castro, Y. Ustate, and L.C. Gómez-Gómez. 2021. Bioestimulación de frijol guajiro y su simbiosis con Rhizobium por ácidos húmicos y Bacillus mycoides. Rev. Bio. Agro. 19(2), 119-134. Doi: https://doi.org/10.18684/bsaa.v19.n2.2021.1608
- Van Oosten, M.J., O. Pepe, S. De Pascale, S. Silletti, and A. Maggio. 2017. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 4(1), 5. Doi: https://doi.org/10.1186/s40538-017-0089-5
- Zandonadi, D.B., C.R.R. Matos, R.N. Castro, R. Spaccini, F.L. Olivares, and L.P. Canellas. 2019. Alkamides: a new class of plant growth regulators linked to humic acid bioactivity. Chem. Biol. Technol. Agric. 6(1), 23. Doi: https://doi.org/10.1186/s40538-019-0161-4
- Zhang, D., D. Duan, Y. Huang, Y. Yang, and Y. Ran. 2017. Composition and structure of natural organic matter through advanced nuclear magnetic resonance techniques. Chem. Biol. Technol. Agric. 4(1), 8. Doi: https://doi.org/10.1186/s40538-017-0086-8
