Biostimulant activity of humic acids derived from goat manure vermicompost and lignite in relation to their structure and interaction with a PGPR strain under semiarid conditions
Abstract
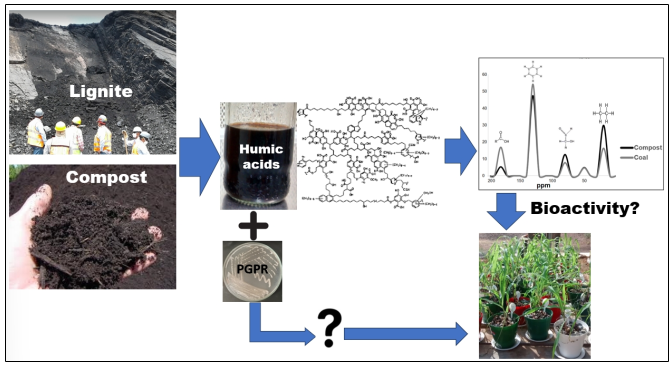
The use of humified organic matter (HOM)-based plant biostimulants and plant growth-promoting rhizobacteria (PGPR) has emerged as a promising approach to enhance agricultural productivity in arid and semiarid environments. However, the bioactivity of humic stimulants varies based on their chemical composition, and the synergistic effects of co-applying these biostimulants remain to be fully elucidated. In this research, we investigated the structural and bioactive characteristics of humic acids derived from goat manure vermicompost (HAVC) and lignite coal (HAC). Additionally, we explored the plant growth-promoting effects of each humic acid (HA) in conjunction with the Bacillus mycoides strain BSC25 (Bm) on corn plants in arid conditions. To assess the relationship between structure and bioactivity, we determined the supramolecular composition of the HAs and evaluated their effectiveness through a corn coleoptile elongation test. Subsequently, we conducted biostimulation tests on maize seedlings in a growth chamber and performed a field-based biostimulation test in a semi-arid region. Notably, HACs exhibited coleoptile elongation at lower concentrations (25-50 mg LC) compared to HAVCs, which required higher concentrations (100-200 mg LC) to achieve the same effect. These outcomes correlated with the supramolecular composition of HAs. The bioactivity of HACs was linked to their oxygen content, aromatic and carboxylic groups, whereas HAVCs' bioactivity was associated with their carbohydrate, aliphatic carbon, and hydrogen content. The application of both HAs, together with Bm, resulted in enhanced corn leaf biomass production in the growth chamber and under field conditions. This effect can be attributed to the hormone-like actions of HA and the PGPR activity of Bm. Interestingly, despite foliar application, HAs displayed bioactivity at the root level, as evidenced by increased root biomass in the field. These results indicate a PGPR effect of Bm that remained unaltered with co-application of HAVC. However, the joint application of Bm-HAC and Bm-HAVC reversed the positive effect of Bm on corn production under field conditions. This outcome likely relates to the hormone-like effects of HA and potential additive effects following Bm inoculation.
Keywords
Bioactive compounds, Zea mays, Bacillus mycoides, Dryland management, Food insecurity, Biotechnology
References
- Aguiar, N.O., E.H. Novotny, A.L. Oliveira, V.M. Rumjanek, F.L. Olivares, and L.P. Canellas. 2013. Prediction of humic acids bioactivity using spectroscopy and multivariate analysis. J. Geochem. Explor. 129, 95-102. Doi: https://doi.org/10.1016/j.gexplo.2012.10.005
- Aguiar, N.O., F.L. Olivares, E.H. Novotny, and L.P. Canellas. 2018. Changes in metabolic profiling of sugarcane leaves induced by endophytic diazotrophic bacteria and humic acids. PeerJ 6, e5445. Doi: https://doi.org/10.7717/peerj.5445
- Ahmad, P., S. Rasool, A. Gul, S.A. Sheikh, N.A. Akram, M. Ashraf, A.M. Kazi, and S. Gucel. 2016. Jasmonates: multifunctional roles in stress tolerance. Front. Plant Sci. 7, 813. Doi: https://doi.org/10.3389/fpls.2016.00813
- Ait Baddi, G., M. Hafidi, V. Gilard, and J.-C. Revel. 2003. Characterization of humic acids produced during composting of olive mill wastes: elemental and spectroscopic analyses (FTIR and 13C-NMR). Agronomie 23(7), 661-666. Doi: https://doi.org/10.1051/agro:2003042
- Araújo, K.V., M. Pittarello, P. Carletti, A.R.M. Campos, and L.B. Dobbss. 2021. Structural characterization and bioactivity of humic and fulvic acids extracted from preserved and degraded Brazilian Cerrado Biomes Soils. Eurasian Soil Sci. 54(1), 16-25. Doi: https://doi.org/10.1134/S1064229322030024
- Bacilio, M., M. Moreno, D.R. Lopez-Aguilar, and Y. Bashan, 2017. Scaling from the growth chamber to the greenhouse to the field: demonstration of diminishing effects of mitigation of salinity in peppers inoculated with plant growth-promoting bacterium and humic acids. Appl. Soil Ecol. 119, 327-338. Doi: https://doi.org/10.1016/j.apsoil.2017.07.002
- Bashan, Y., B.G. Salazar, M. Moreno, B.R. Lopez, and R.G. Linderman. 2012. Restoration of eroded soil in the Sonoran Desert with native leguminous trees using plant growth-promoting microorganisms and limited amounts of compost and water. J. Environ. Manage. 102, 26-36. Doi: https://doi.org/10.1016/j.jenvman.2011.12.032
- Beleño-Carrillo, J., L. Gómez-Gómez, and N.O. Valero-Valero. 2022. Bacillus mycoides y ácidos húmicos como bioestimulantes de fríjol caupí bajo estrés por salinidad. Rev. U.D.C.A Act. Div. Cient. 25(2), e1974. Doi: https://doi.org/10.31910/rudca.v25.n2.2022.1974
- Blouin, M. 2018. Chemical communication: An evidence for co-evolution between plants and soil organisms. Appl. Soil Ecol. 123, 409-415. Doi: https://doi.org/10.1016/j.apsoil.2017.10.028
- Bratkova, S., K. Nikolova, P. Genova, and A. Angelov. 2021. Use of plant growth-promoting bacteria and humic acids for phytostabilization of acid-generating mining wastes. Res Sq. Preprint v1. Doi: https://doi.org/10.21203/rs.3.rs-550365/v1
- Brito-Campo, H.L., M.F. Ayala-Santamaría, K.J. Barros-Escalante, J.G. Cubillos-Hinojosa, M.F. Pantoja-Guerra, N.O. Valero, and L. Gómez. 2022. PGPR activity of coal solubilizing bacteria. Rev. Fac. Agron.(LUZ) 39(2), e223932. Doi: https://doi.org//10.47280/RevFacAgron(LUZ).v39.n2.10
- Bulgari, R., G. Franzoni, and A. Ferrante. 2019. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 9(6), 306. Doi: https://doi.org/10.3390/agronomy9060306
- Canellas, L.P., N.O.A. Canellas, L.E.S.S. Irineu, F.L. Olivares, and A. Piccolo. 2020. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 7(1), 12. Doi: https://doi.org/10.1186/s40538-020-00178-4
- Canellas, L.P., D.J. Dantas, N.O. Aguiar, L.E.P. Peres, A. Zsögön, F.L. Olivares, L.B. Dobbss, A.R. Façanha, A. Nebibioso, and A. Piccolo. 2011. Probing the hormonal activity of fractionated molecular humic components in tomato auxin mutants. Ann. Appl. Biol. 159(2), 202-211. Doi: https://doi.org/10.1111/j.1744-7348.2011.00487.x
- Canellas, L.P., A. Piccolo, L.B. Dobbss, R. Spaccini, F.L. Olivares, D.B. Zandonadi, and A.R. Façanha. 2010. Chemical composition and bioactivity properties of size-fractions separated from a vermicompost humic acid. Chemosphere 78(4), 457-466. Doi: https://doi.org/10.1016/j.chemosphere.2009.10.018
- Canellas, L.P. and F.L. Olivares. 2014. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 1(1), 3. Doi: https://doi.org/10.1186/2196-5641-1-3
- Canellas, L.P., F.L. Olivares, N.O.A. Canellas, P. Mazzei, and A. Piccolo. 2019. Humic acids increase the maize seedlings exudation yield. Chem. Biol. Technol. Agric. 6(1), 3. Doi: https://doi.org/10.1186/s40538-018-0139-7
- Canellas, L.P., F.L. Olivares, A.L. Okorokova-Façanha, and A.R. Façanha. 2002. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol. 130(4), 1951-1957. Doi: https://doi.org/10.1104/pp.007088
- Cavagnaro, T.R. 2014. Impacts of compost application on the formation and functioning of arbuscular mycorrhizas. Soil Biol. Biochem. 78, 38-44. Doi: https://doi.org/10.1016/j.soilbio.2014.07.007
- Chang, R.R., R. Mylotte, M.H.B. Hayes, R. Mclnerney, and Y.M. Tzou. 2014. A comparison of the compositional differences between humic fractions isolated by the IHSS and exhaustive extraction procedures. Naturwissenschaften 101(3), 197-209. Doi: https://doi.org/10.1007/s00114-013-1140-4
- Conklin, P.A., J. Strable, S. Li, and M.J. Scanlon. 2019. On the mechanisms of development in monocot and eudicot leaves. New Phytol. 221(2), 706-724. Doi: https://doi.org/10.1111/nph.15371
- Da Piedade Melo, A., F.L. Olivares, L.O. Médici, A. Torres-Neto, L.B. Dobbss, and L.P. Canellas. 2017. Mixed rhizobia and Herbaspirillum seropedicae inoculations with humic acid-like substances improve water-stress recovery in common beans. Chem. Biol. Technol. Agric. 4(1), 6. Doi: https://doi.org/10.1186/s40538-017-0090-z
- De Sanfilippo, E.C., J.A. Argüello, G. Abdala, and G.A. Orioli. 1990. Content of auxin-inhibitor-and gibberellin-like substances in humic acids. Biol. Plant. 32(5), 346-351. Doi: https://doi.org/10.1007/BF02898497
- Du Jardin, P. 2015. Plant biostimulants: definition, concept, main categories and regulation. Sci. Hortic. 196, 3-14. Doi: https://doi.org/10.1016/j.scienta.2015.09.021
- Elmongy, M.S., X. Wang, H. Zhou, and Y. Xia. 2020. Humic acid and auxins induced metabolic changes and differential gene expression during adventitious root development in azalea microshoots. HortScience 55(6), 926-935. Doi: https://doi.org/10.21273/HORTSCI14885-20
- FAO. 2022. Agricultura de Conservación. In: https://www.fao.org/3/cb8350es/cb8350es.pdf; consulted: February, 2023.
- Fischer, T. 2017. Humic supramolecular structures have polar surfaces and unpolar cores in native soil. Chemosphere 183, 437-443. Doi: https://doi.org/10.1016/j.chemosphere.2017.05.125
- García A.C., L.G. Ambrosio de Souza, M.G. Pereira, R.N. Castro, J.M. García-Mina, E. Zonta, F.J.G. Lisboa, and R.L.L. Berbara. 2016a. Structure-property-function relationship in humic substances to explain the biological activity in plants. Sci. Rep. 6(1), 20798. Doi: https://doi.org/10.1038/srep20798
- García, A.C., L. Azevedo, F. Guridi, V.M. Rumjanek, R.N. Castro, F.S. Santos, L.G.A. Souza, and R.L.L. Berbara. 2014. Potentialities of vermicompost humic acids to alleviate water stress in rice plants (Oryza sativa L.). J. Geochem. Explor. 136, 48-54. Doi: https://doi.org/10.1016/j.gexplo.2013.10.005
- García, A.C., L. Azevedo, L.G.A. Souza, O.C. Huertas, A. Zonta, E.T. Martins, J.M. García-Mina, and R.L.L. Berbara. 2016b. Vermicompost humic acids modulate the accumulation and metabolism of ROS in rice plants. J. Plant Physiol. 192, 56-63. Doi: https://doi.org/10.1016/j.jplph.2016.01.008
- García, A.C., O.C. Huertas, D. Martínez, V.S. Almeida, L.P. Canellas, J.M. García-Mina, and R.L.L. Berbara. 2018. Structure-function relationship of vermicompost humic fractions for use in agriculture. J. Soils Sediments 18(4), 1365-1375. Doi: https://doi.org/10.1007/s11368-016-1521-3
- García, A.C., T.A. Van Tol de Castro, L.A. Santos, O.C.H. Tavares, R.N. Castro, R.C.L. Berbara, and J.M. García-Mina. 2019. Structure-property-function relationship of humic substances in modulating the root growth of plants: a review. J. Environ. Qual. 48(6), 1622-1632. Doi: https://doi.org/10.2134/jeq2019.01.0027
- Giannouli, A., S. Kalaitzidis, G. Siavalas, A. Chatziapostolou, K. Christanis, S. Papazisimou, C. Papanicolou, and A. Foscolos. 2009. Evaluation of greek low-rank coals as potential raw material for the production of soil amendments and organic fertilizers. Int. J. Coal Geol. 77(3-4), 383-393. Doi: https://doi.org/10.1016/j.coal.2008.07.008
- Gualdrón, R. 2011. Cerrejón: hacia la rehabilitación de las tierras intervenidas por la minería a cielo abierto. El Cerrejón, Bogota.
- Jindo, K., F.L. Olivares, D.J.P. Malcher, M.A. Sánchez-Monedero, C. Kempenaar, and L.P. Canellas. 2020. From lab to field: role of humic substances under open-field and greenhouse conditions as biostimulant and biocontrol agent. Front. Plant Sci. 11, 426. Doi: https://doi.org/10.3389/fpls.2020.00426
- Li, J., J. Wang, H. Liu, C.A. Macdonald, and B.K. Singh. 2022. Application of microbial inoculants significantly enhances crop productivity: a meta-analysis of studies from 2010 to 2020. J. Sustain. Agric. Environ. 1(3), 216-225. Doi: 10.1002/sae2.12028
- McSteen, P. 2010. Auxin and monocot development. Cold Spring Harb. Perspect. Biol. 2010(2), a001479. Doi: https://doi.org/10.1101/cshperspect.a001479
- Mora, V., E. Bacaicoa, R. Baigorri, A.M. Zamarreño, and J.M. García-Mina. 2014. NO and IAA key regulators in the shoot growth promoting action of humic acid in Cucumis sativus L. J. Plant Growth Regul. 33(2), 430-439. Doi: https://doi.org/10.1007/s00344-013-9394-9
- Muscolo, A., F. Bovalo, F. Gionfriddo, and S. Nardi. 1999. Earthworm humic matter produces auxin-like effects on Daucus carota cell growth and nitrate metabolism. Soil Biol. Biochem. 31(9), 1303-1311. Doi: https://doi.org/10.1016/S0038-0717(99)00049-8
- Muscolo, A., D. Pizzeghello, O. Francioso, S. Sanchez, and S. Nardi. 2020. Effectiveness of humic substances and phenolic compounds in regulating plant-biological functionality. Agronomy 10(10), 1553. Doi: https://doi.org/10.3390/agronomy10101553
- Muscolo, A., M. Sidari, E. Attinà, O. Francioso, V. Tugnoli, and S. Nardi. 2007a. Biological activity of humic substances is related to their chemical structure. Soil Sci. Soc. Am. J. 71(1), 75-85. Doi: https://doi.org/10.2136/sssaj2006.0055
- Muscolo, A., M. Sidari, O. Francioso, V. Tugnoli, and S. Nardi. 2007b. The auxin-like activity of humic substances is related to membrane interactions in carrot cell cultures. J. Chem. Ecol. 33(1), 115-129. Doi: https://doi.org/10.1007/s10886-006-9206-9
- Muscolo, A., M. Sidari, and S. Nardi. 2013. Humic substance: relationship between structure and activity. Deeper information suggests univocal findings. J. Geochem. Explor. 129, 57-63. Doi: https://doi.org/10.1016/j.gexplo.2012.10.012
- Nardi, S., D. Pizzeghello, and A. Ertani. 2018. Hormone-like activity of the soil organic matter. Appl. Soil Ecol. 123, 517-520. Doi: https://doi.org/10.1016/j.apsoil.2017.04.020
- Nardi, S., D. Pizzeghello, A. Muscolo, and A. Vianello. 2002. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 34(11), 1527-1536. Doi: https://doi.org/10.1016/S0038-0717(02)00174-8
- Nardi, S., D. Pizzeghello, M. Schiavon, and A. Ertani. 2016. Plant biostimulants: physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 73(1), 18-23. Doi: https://doi.org/10.1590/0103-9016-2015-0006
- Nardi, S., M. Schiavon, and O. Francioso. 2021. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 26(8), 2256. Doi: https://doi.org/10.3390/molecules26082256
- Nebbioso, A. and A. Piccolo. 2011. Basis of a humeomics science: chemical fractionation and molecular characterization of humic biosuprastructures. Biomacromolecules 12(4), 1187-1199. Doi: https://doi.org/10.1021/bm101488e
- Nelissen, H., N. Gonzalez, and D. Inzé. 2016. Leaf growth in dicots and monocots: so different yet so alike. Curr. Opin. Plant Biol. 33, 72-76. Doi: https://doi.org/10.1016/j.pbi.2016.06.009
- Nunes, R.O., G.A. Domociano, W.S. Alves, A.C.A. Melo, F.C.S. Nogueira, L.P. Canellas, F.L. Olivares, R.B. Zingali, and M.R. Soares. 2019. Evaluation of the effects of humic acids on maize root architecture by label-free proteomics analysis. Sci. Rep. 9(1), 12019. Doi: https://doi.org/10.1038/s41598-019-48509-2
- Ojwang’ L.M. and R.L. Cook. 2013. Environmental conditions that influence the ability of humic acids to induce permeability in model biomembranes. Environ. Sci. Technol. 47(15), 8280-8287. Doi: https://doi.org/10.1021/es4004922
- Olaetxea, M., V. Mora, E. Bacaicoa, R. Baigorri, M. Garnica, M. Fuentes, A.M. Zamarreño, L. Spíchal, and J.M. GArcía-Mina. 2019. Root ABA and H+‐ATPase are key players in the root and shoot growth‐promoting action of humic acids. Plant Direct 3(10), e00175. Doi: https://doi.org/10.1002/pld3.175
- Pantoja-Guerra, M. and N. Valero-Valero. 2020. Design of a bio-conditioner prototype for the treatment of degraded soils: biomass production and lignite formulation for Microbacterium sp. CSB3. Chem. Biol. Technol. Agric. 7, 3. Doi: https://doi.org/10.1186/s40538-019-0167-y
- Pantoja-Guerra, M., N. Valero-Valero, and C.A. Ramírez. 2023. Total auxin level in the soil–plant system as a modulating factor for the effectiveness of PGPR inocula: a review. Chem. Biol. Technol. Agric. 10, 6. Doi: https://doi.org/10.1186/s40538-022-00370-8
- Pizzeghello, D., O. Francioso, A. Ertani, A. Muscolo, and S. Nardi. 2013. Isopentenyladenosine and cytokinin-like activity of different humic substances. J. Geochem. Explor. 129, 70-75. Doi: https://doi.org/10.1016/j.gexplo.2012.10.007
- Pizzeghello, D., M. Schiavon, O. Francioso, F.D. Vecchia, A. Ertani, and S. Nardi. 2020. Bioactivity of size-fractionated and unfractionated humic substances from two forest soils and comparative effects on N and S metabolism, nutrition, and root anatomy of Allium sativum L. Front. Plant Sci. 11, 1203. Doi: https://doi.org/10.3389/fpls.2020.01203
- Reeves, T.G., G. Thomas, and G. Ramsay. 2016. Save and grow in practice: maize, rice, wheat. A guide to sustainable cereal production. FAO, Rome.
- Ritchie, J.D. and E.M. Perdue. 2008. Analytical constraints on acidic functional groups in humic substances. Org. Geochem. 39(6), 783-799. Doi: https://doi.org/10.1016/j.orggeochem.2008.03.003
- Rojas, D., M. Contreras, and G. Santoyo. 2013. Mecanismos de estimulación del crecimiento vegetal en bacterias del género Bacillus. Biológicas 15(2), 36-41.
- Roomi, S., A. Masi, G.B. Conselvan, S. Trevisan, S. Quaggiotti, M. Pivato, G. Arrigoni, T. Yasmin, and P. Carletti. 2018. Protein profiling of Arabidopsis roots treated with humic substances: insights into the metabolic and interactome networks. Front. Plant Sci. 9, 1812. Doi: https://doi.org/10.3389/fpls.2018.01812
- Scaglia, B., R.R. Nunes, M.O.O. Rezende, F. Tambone, and F. Adani. 2016. Investigating organic molecules responsible of auxin-like activity of humic acid fraction extracted from vermicompost. Sci. Total Environ. 562, 289-295. Doi: https://doi.org/10.1016/j.scitotenv.2016.03.212
- Shah, Z.H., H.F. Rehman, T. Akhtar, H. Alsamadany, B.T. Hamooh, T. Mujtaba, I. Daur, Y.A. Zahrani, H.A.S. Alzahrani, S. Ali, S.H. Yang, and G. Chung. 2018. Humic substances: determining potential molecular regulatory processes in plants. Front. Plant Sci. 9, 263. Doi: https://doi.org/10.3389/fpls.2018.00263
- Sharma, H.S.S., C. Selby, E. Carmichael, C. McRoberts, J.R. Rao, P. Ambriosio, M. Chiurazzi, M. Pucci, and T. Martin. 2016. Physicochemical analyses of plant biostimulant formulations and characterisation of commercial products by instrumental techniques. Chem. Biol. Technol. Agric. 3, 13. Doi: https://doi.org/10.1186/s40538-016-0064-6
- Spaccini, R. and A. Piccolo. 2009. Molecular characteristics of humic acids extracted from compost at increasing maturity stages. Soil Biol. Biochem. 41(6), 1164-1172. Doi: https://doi.org/10.1016/j.soilbio.2009.02.026
- Tahiri, A., A. Richel, J. Destain, P. Druart, P. Thonart, and M. Ongena. 2016. Comprehensive comparison of the chemical and structural characterization of landfill leachate and leonardite humic fractions. Anal. Bioanal. Chem. 408(7), 1917-1928. Doi: https://doi.org/10.1007/s00216-016-9305-6
- Tejera-Hernández, B., M.M. Rojas-Badia, and M. Heydrich-Pérez. 2011. Potencialidades del género Bacillus en la promoción del crecimiento vegetal y el control biológico de hongos fitopatógenos. Rev. CENIC Cienc. Biol. 42(3), 131-138.
- Trevisan, S., O. Francioso, S. Quaggiotti, and S. Nardi. 2010. Humic substances biological activity at the plant-soil interface. Plant Signal. Behav. 5(6), 635-643. Doi: https://doi.org/10.4161/psb.5.6.11211
- Valero, N., L. Gómez, M. Pantoja, and R. Ramírez. 2014. Production of humic substances through coal-solubilizing bacteria. Brazilian J. Microbiol. 45(3), 911-918. Doi: https://doi.org/10.1590/s1517-83822014000300021
- Valero, N.O., L.C. Gómez, and L.M. Melgarejo. 2018b. Supramolecular characterization of humic acids obtained through the bacterial transformation of a low rank coal. J. Braz. Chem. Soc. 29(9), 1842-1853. Doi: https://doi.org/10.21577/0103-5053.20180060
- Valero, N., L.M. Melgarejo, and R. Ramírez. 2016. Effect of low-rank coal inoculated with coal solubilizing bacteria on edaphic materials used in post-coal-mining land reclamation: a greenhouse trial. Chem. Biol. Technol. Agric. 3, 20. Doi: https://doi.org/10.1186/s40538-016-0068-2
- Valero, N., J.A. Salgado, and D. Corzo. 2018a. Metodología sencilla para evaluar bioactividad de ácidos húmicos obtenidos de lignito mediante extracción alcalina y bacterias solubilizadoras de carbón. Inf. Tecnol. 29(4), 75-82. Doi: https://doi.org/10.4067/S0718-07642018000400075
- Valero-Valero, N.-O., C.M. Vergel-Castro, Y. Ustate, and L.C. Gómez-Gómez. 2021. Bioestimulación de frijol guajiro y su simbiosis con Rhizobium por ácidos húmicos y Bacillus mycoides. Rev. Bio. Agro. 19(2), 119-134. Doi: https://doi.org/10.18684/bsaa.v19.n2.2021.1608
- Van Oosten, M.J., O. Pepe, S. De Pascale, S. Silletti, and A. Maggio. 2017. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 4(1), 5. Doi: https://doi.org/10.1186/s40538-017-0089-5
- Zandonadi, D.B., C.R.R. Matos, R.N. Castro, R. Spaccini, F.L. Olivares, and L.P. Canellas. 2019. Alkamides: a new class of plant growth regulators linked to humic acid bioactivity. Chem. Biol. Technol. Agric. 6(1), 23. Doi: https://doi.org/10.1186/s40538-019-0161-4
- Zhang, D., D. Duan, Y. Huang, Y. Yang, and Y. Ran. 2017. Composition and structure of natural organic matter through advanced nuclear magnetic resonance techniques. Chem. Biol. Technol. Agric. 4(1), 8. Doi: https://doi.org/10.1186/s40538-017-0086-8
