Antimicrobial activity of Bothrops asper and Porthidium nasutum venom on purple passion fruit (Passiflora edulis f. edulis) phytopathogens
Abstract
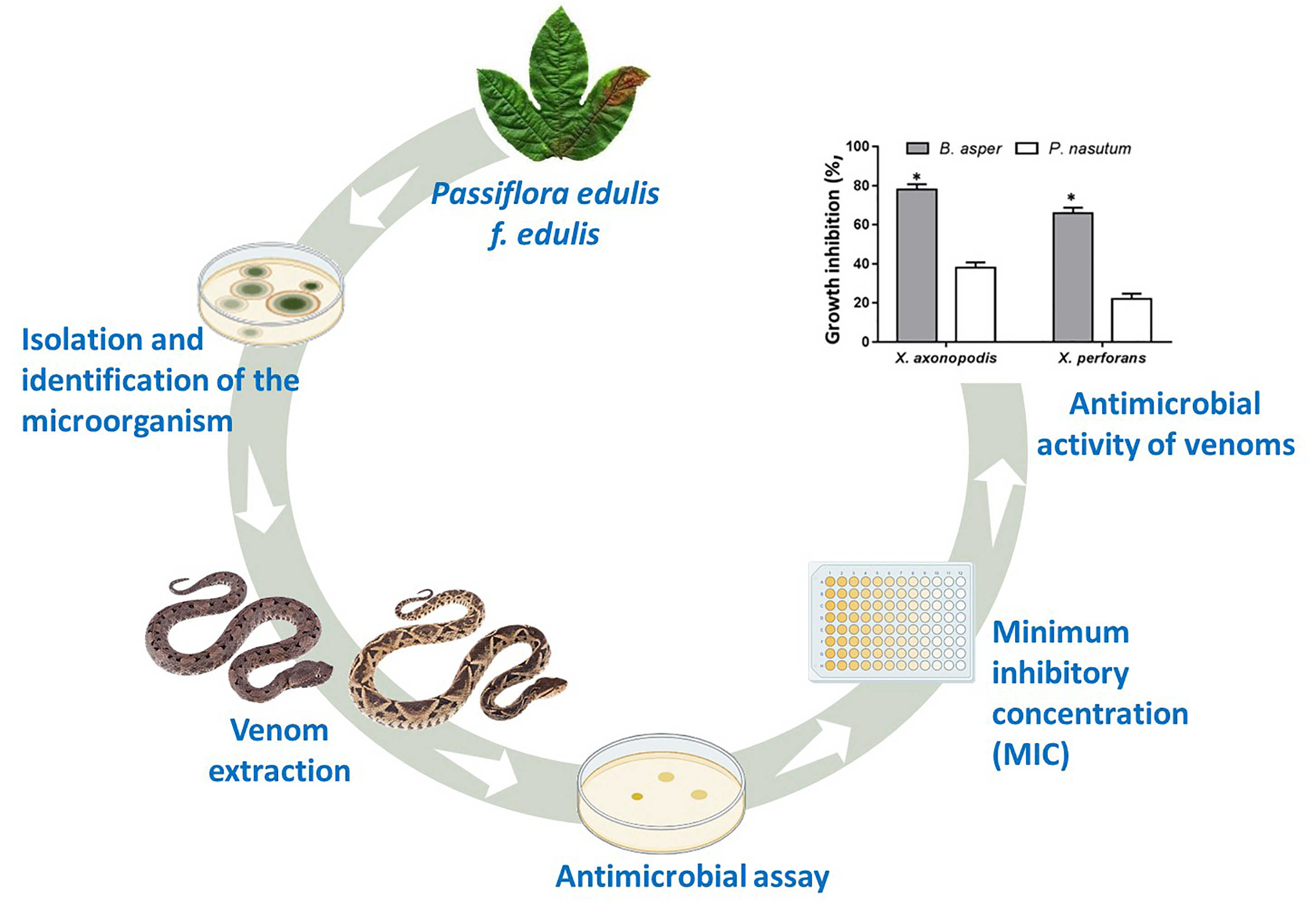
The most important pathogens causing high damage to fruit and vegetable crops are bacteria and fungi; among them Xanthomonas and Fusarium respectively are in the phytopathogens responsible of diseases in those crops. In Colombia, the crop of Passiflora edulis f. edulis occupies a position preferential within international market. However different pathologies affect their production, one of them is the infectious diseases and are caused by microorganisms as Xanthomonas and Fusarium. The control includes application of pesticides or antibiotics, but to some of them the microorganisms have develop resistance is widespread, and others are restricted associated to the impact on the environment and effects in animal and human healthy. Then find other alternatives is necessary, in this work the snake venoms of the vipers Bothrops asper and Porthidium nasutum were evaluated in ability to inhibit growth of these microorganism isolated of purple passion fruit crops with typical symptoms of diseases associated with infections by microorganisms. For these antimicrobial assays were performed in agar culture and broth microdilution. The results showed growth inhibition of 80% to B. asper against Xanthomonas axonopodis and 70% Xanthomonas perforans. The minimum inhibitory concentration was 31,2 µg mL-1 to B. asper and 500 µg mL-1 to P. nasutum to both bacteria. Nevertheless, the activity against F. oxysporum was low. The isolation of compounds responsible of this activity will contribute to the development of new molecules for antimicrobial agents against phytopathogens affecting purple passion fruit crops.
Keywords
Snake venoms, Plant infectious diseases, Xanthomones, Fusarium, Bioprospecting
References
- Abrahamian, P., J.M. Klein-Gordon, J.B. Jones, and G.E. Vallad. 2021. Epidemiology, diversity, and management of bacterial spot of tomato caused by Xanthomonas perforans. Appl. Microbiol. Biotechnol. 105(16-17), 6143-6158. Doi: https://doi.org/10.1007/s00253-021-11459-9
- Alape-Girón A., Flores-Díaz M., Sanz L., Madrigal M., Escolano J., Sasa M., Calvete J.J. 2009. Studies on the venom proteome of Bothrops asper: perspectives and applications. Toxicon 54(7), 938-948. Doi: https://doi.org/10.1016/j.toxicon.2009.06.011
- Alves E., A. Simoes, and M.R. Domingues. 2021. Fruit seeds and their oils as promising sources of value-added lipids from agro-industrial byproducts: oil content, lipid composition, lipid analysis, biological activity and potential biotechnological applications. Crit. Rev. Food Sci. Nutr. 61(8), 1305-1339. Doi: https://doi.org/10.1080/10408398.2020.1757617
- Barbosa, P.S., A.M. Martins, A. Havt, D.O. Toyama, J.S. Evangelista, D.P. Ferreira, P.P. Joazeiro, L.O. Beriam, M.H. Toyama, M.C. Fonteles, and H.S. Monteiro. 2005. Renal and antibacterial effects induced by myotoxin I and II isolated from Bothrops jararacussu venom. Toxicon 46(4), 376-86. Doi: https://doi.org/10.1016/j.toxicon.2005.04.024
- Benítez, S, W. De León, and L. Farfán-Caceres. 2011. Proceso infectivo de la mancha de aceite causada por Xanthomonas axonopodis en gulupa (Passiflora edulis Sims). Fitopatol. Colomb. 35(2), 57-62.
- Benítez, S. and L. Hoyos. 2009. Sintomatología asociada a bacteriosis en zonas productoras de gulupa (Passifora edulis Sims.) en Colombia. Rev. Colomb. Cienc. Hortic. 3, 276-280. Doi: https://doi.org/10.17584/rcch.2009v3i2.1218
- Carmona-Hernández, J.C., G. Taborda-Ocampo, J.C. Valdez, B.W. Bolling, and C.H. González-Correa. 2019. Polyphenol extracts from three Colombian Passifloras (passion fruits) prevent inflammation-induced barrier dysfunction of caco-2 cells. Molecules 24(24), 4614. Doi: https://doi.org/10.3390/molecules24244614
- Charvat, R.A., R.M. Strobel, M.A. Pasternak, S.M. Klass, and J.L. Rheubert. 2018. Analysis of snake venom composition and antimicrobial activity. Toxicon 150, 151-167. Doi: https://doi.org/10.1016/j.toxicon.2018.05.016
- Corrêa, R.C.G., R.M. Peralta, C.W.I. Haminiuk, G.M. Maciel, A. Bracht, and C.F.R. Ferreira. 2016. The past decade findings related with nutritional composition, bioactive molecules and biotechnological applications of Passiflora spp. (passion fruit). Trend. Food Sci. Technol. 58, 79-95. Doi: https://doi.org/10.1016/j.tifs.2016.10.006
- de Barros, E., R.M. Gonçalves, M.H. Cardoso, N.C. Santos, O.L. Franco, and E.S. Cândido. 2019. Snake cenom Cathelicidins as natural antimicrobial peptides. Front. Pharmacol. 10, 1415. Doi: https://doi.org/10.3389/fphar.2019.01415
- Falcao, C.B. and G. Radis-Baptista. 2020. Crotamine and crotalicidin, membrane active peptides from Crotalus durissus terrificus rattlesnake venom, and their structurally-minimized fragments for applications in medicine and biotechnology. Peptides 126, 170234. Doi: https://doi.org/10.1016/j.peptides.2019.170234
- Farfán L.M., S. Benítez, and L.M. Hoyos-Carvajal. 2014. Purple passion fruit bacteria sensitivity to antibiotics and copper products. Rev. Colomb. Cienc. Hortic. 8(1), 20-23. Doi: https://doi.org/10.17584/rcch.2014v8i1.2797
- Fernández, R.J. and C.L. Suárez. 2009. Antagonism in vitro of Trichoderma harzianum Rifai against Fusarium oxysporum Schlecht f. sppassiflorae in passion fruit (Passiflora edulis Sims var. Flavicarpa) from Colombia bananera zone municipality. Rev. Fac. Nal. Agr. Medellín 62(1), 4743-4748.
- Fischer, G., A. Parra-Coronado, and H.E. Balaguera-López. 2022. Altitude as a determinant of fruit quality with emphasis on the Andean tropics of Colombia. A review. Agron. Colomb. 40(2), 212–227. Doi: https://doi.org/10.15446/agron.colomb.v40n2.101854
- Fonseca, A.M.A, M.V. Geraldi, M.R.M. Junior, A.J.D. Silvestre, and S.M. Rocha. 2022. Purple passion fruit (Passiflora edulis f. edulis): a comprehensive review on the nutritional value, phytochemical profile and associated health effects. Food Res. Int. 160, 111665. Doi: https://doi.org/10.1016/j.foodres.2022.111665
- Gomes, V.M., A.O. Carvalho, M. Da Cunha, M.N. Keller, C. Jr Bloch, P. Deolindo, and E.W. Alves. 2005. Purification and characterization of a novel peptide with antifungal activity from Bothrops jararaca venom. Toxicon 45(7), 817-827. Doi: https://doi.org/10.1016/j.toxicon.2004.12.011
- He, X., F. Luan, Y. Yang, Z. Wang, Z. Zhao, J. Fang, M. Wang, M. Zuo, and Y. Li. 2020. Passiflora edulis: an insight into current researches on phytochemistry and pharmacology. Front. Pharmacol. 11, 617. Doi: https://doi.org/10.3389/fphar.2020.00617
- Hettiarachchi, H.A.C.O, N.N.G. Chiranthika, G. Janarny, and K.D.P.P. Gunathilake. 2022. Passion fruit (Passiflora edulis). In: Handbook of phytonutrients in indigenous fruits and vegetables. Doi: https://doi.org/10.1079/9781789248067.0016
- Joy, P.P. and C.G. Sherin. 2016. Diseases of passion fruit (Passiflora edulis) and their management. pp. 453-470. In: A. Kumar Pandey, and P. Mall (ed.). Insect pest’s managements of fruit crops. Biotech Books.
- Lomonte, B., P. Rey-Suárez, W.C. Tsai, Y. Angulo, M. Sasa, J.M. Gutiérrez, and J.J. Calvete. 2012. Snake venomics of the pit vipers Porthidium nasutum, Porthidium ophryomegas, and Cerrophidion godmani from Costa Rica: toxicological and taxonomical insights. J. Proteomics. 75(5), 1675-1689. Doi: https://doi.org/10.1016/j.jprot.2011.12.016
- Lozano-Montaña P.A., F. Sarmiento, L.M. Mejía-Sequera, F. Álvarez-Flórez, and L.M. Melgarejo. 2021. Physiological, biochemical and transcriptional responses of Passiflora edulis Sims f. edulis under progressive drought stress. Sci. Hort. 275, Doi: https://doi.org/10.1016/j.scienta.2020.109655
- Mansfield, J., S. Genin, S. Magori, V. Citovsky, M. Sriariyanum, P. Ronald, M. Dow, V. Verdier, S.V. Beer, M.A. Machado, I. Toth, G. Salmond, and G.D. Foster. 2012. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13(6), 614-29. Doi: https://doi.org/10.1111/j.1364-3703.2012.00804.x
- Memariani, H., D. Shahbazzadeh, R. Ranjbar, M. Behdani, M. Memariani, K. Pooshang Bagheri. 2017. Design and characterization of short hybrid antimicrobial peptides from pEM-2, mastoparan-VT1, and mastoparan-B. Chem. Biol. Drug. Des. 89(3), 327-338. Doi: https://doi.org/10.1111/cbdd.12864
- Munhoz, C.F., B. Weiss, L.R. Hanai, M.I. Zucchi, M.H. Fungaro, A.L. Oliveira, C.B. Monteiro-Vitorello, and M.L. Vieira. 2011. Genetic diversity and a PCR-based method for Xanthomonas axonopodis detection in passion fruit. Phytopathology 101(4), 416-424. Doi: https://doi.org/10.1094/PHYTO-06-10-0169
- Murillo, L.A., C.Y. Lan, N.M. Agabian, S. Larios, and B. Lomonte. 2007. Fungicidal activity of a phospholipase-A2-derived synthetic peptide variant against Candida albicans. Rev. Esp. Quimioter. 20(3), 330-333.
- Nakayinga, R., A. Makumi, V. Tumuhaise, and W. Tinzaara. 2021. Xanthomonas bacteriophages: a review of their biology and biocontrol applications in agriculture. BMC Microbiol. 21(1), 291. Doi: https://doi.org/10.1186/s12866-021-02351-7
- Nunes-Edos, S., M.A. Souza, A.F. Vaz, G.M. Santana, F.S. Gomes, L.C. Coelho, P.M. Paiva, R.M. Silva, R.A. Silva-Lucca, M.L. Oliva, M.C. Guarnieri, and M.T. Correia. 2011. Purification of a lectin with antibacterial activity from Bothrops leucurus snake venom. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 159(1), 57-63. Doi: https://doi.org/10.1016/j.cbpb.2011.02.001
- Oliveira, S.C., F.V. Fonseca, E. Antunes, E.A. Camargo, R.P. Morganti, R. Aparício, D.O. Toyama, L.O. Beriam, E.V. Nunes, B.S. Cavada, C.S. Nagano, A.H. Sampaio, K.S. Nascimento, and M.H. Toyama. 2008. Modulation of the pharmacological effects of enzymatically-active PLA2 by BTL-2, an isolectin isolated from the Bryothamnion triquetrum red alga. BMC Biochem. 9, 16. Doi: https://doi.org/10.1186/1471-2091-9-16
- Pérez-Peinado, C., S. Defaus, and D. Andreu. 2020. Hitchhiking with nature: snake venom peptides to fight cancer and superbugs. Toxins (Basel) 12(4), 255. Doi: https://doi.org/10.3390/toxins12040255
- Rádis-Baptista, G., F.B. Moreno, L.L. Nogueira, A.M. Martins, D. Toyama, M.H. Toyama, B.S. Cavada, W.F. Azevedo Jr, and T. Yamane. 2006. Crotacetin, a novel snake venom C-type lectin homolog of convulxin, exhibits an unpredictable antimicrobial activity. Cell Biochem. Biophys. 44(3), 412-423. Doi: https://doi.org/10.1385/cbb:44:3:412
- Rey-Suárez, P., C. Acosta, U. Torres, M. Saldarriaga-Córdoba, B. Lomonte, and V. Núñez. 2018. MipLAAO, a new L-amino acid oxidase from the redtail coral snake Micrurus mipartitus. Peer J. 6, e4924. Doi: https://doi.org/10.7717/peerj.4924
- Rodríguez-Polanco, E., P. Bermeo, J. Segura-Amaya, and E. Parra-Alferes. 2022. Caracterización y tipificación de los sistemas de producción de gulupa (Passiflora edulis f. edulis Sims) de las regiones Norte y Centro - Occidente de Tolima. Rev. Invest. Agr. Ambient. 13(1), 89-107. Doi: https://doi.org/10.22490/21456453.4583
- Samy, R.P., M. Kandasamy, P. Gopalakrishnakone, B.G. Stiles, E.G. Rowan, D. Becker, M.K. Shanmugam, G. Sethi, and V.T. Chow. 2014. Wound healing activity and mechanisms of action of an antibacterial protein from the venom of the eastern diamondback rattlesnake (Crotalus adamanteus). PLoSOne 9(2), e80199. Doi: https://doi.org/10.1371/journal.pone.0080199
- Santamaría, C., S. Larios, Y. Angulo, J. Pizarro-Cerda, J.P. Gorvel, E. Moreno, and B. Lomonte. 2005. Antimicrobial activity of myotoxic phospholipases A2 from crotalid snake venoms and synthetic peptide variants derived from their C-terminal region. Toxicon 45(7), 807-815. Doi: https://doi.org/10.1016/j.toxicon.2004.09.012
- Seepe, H.A., W. Nxumalo, and S.O. Amoo. 2021. Natural products from medicinal plants against phytopathogenic Fusarium species: current research endeavours, challenges and prospects. Molecules 26(21), 6539. Doi: https://doi.org/10.3390/molecules26216539
- Sepúlveda, M., D. Cardona, Y. Gallo, M. Higuita, P. Gutiérrez, and M. Marín. 2022. Virome analysis for identification of viruses associated with asymptomatic infection of purple passion fruit (Passiflora edulis f. edulis) in Colombia. J. Hort. Sci. Biotechnol. 97(2), 187-200. Doi: https://doi.org/10.1080/14620316.2021.1973583
- Shah, I.H., M.A. Manzoor, I.A. Sabir, M. Ashraf, S. Gulzar, L. Chang, and Y. Zhang. 2022. A green and environmental sustainable approach to synthesis the Mn oxide nanomaterial from Punica granatum leaf extracts and its in vitro biological applications. Environ. Monit. Assess. 194(12), 921. Doi: https://doi.org/10.1007/s10661-022-10606-7
- Shamsi, T.N., R. Parveen, and S. Fatima. 2016. Characterization, biomedical and agricultural applications of protease inhibitors: a review. Int. J. Biol. Macromol. 91, 1120-1133. Doi: https://doi.org/10.1016/j.ijbiomac.2016.02.069
- Syed, A.B. S.F. Rahman, E. Singh, C.M.J. Pieterse, and P.M. Schenk. 2018.Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 267, 102-111. Doi: https://doi.org/10.1016/j.plantsci.2017.11.012
- Toyama, M.H., D. Toyama, L.F. Passero, M.D. Laurenti, C.E. Corbett, T.Y. Tomokane, F.V. Fonseca, E. Antunes, P.P. Joazeiro, L.O. Beriam, M.A. Martins, H.S. Monteiro, and M.C. Fonteles. 2006. Isolation of a new L-amino acid oxidase from Crotalus durissus cascavella venom. Toxicon 47(1), 47-57. Doi: https://doi.org/10.1016/j.toxicon.2005.09.008
- Vargas, L.J., M. Londoño, J.C. Quintana, C. Rua, C. Segura, B. Lomonte, and V. Núñez. 2012. An acidic phospholipase A₂ with antibacterial activity from Porthidium nasutum snake venom. Comp. Biochem. Physiol. B Biochem. Mol, Biol. 161(4), 341-347. Doi: https://doi.org/10.1016/j.cbpb.2011.12.010
- Yu, H.Y., K.C. Huang, B.S. Yip, C.H. Tu, H.L. Chen, H.T. Cheng, and J.W. Cheng. 2010. Rational design of tryptophan-rich antimicrobial peptides with enhanced antimicrobial activities and specificities. Chembiochem. 11(16), 2273-2282. Doi: https://doi.org/10.1002/cbic.201000372
