Exogenous brassinosteroids application in purple passion fruit plants grafted onto a sweet calabash passion fruit rootstock and under water stress
Abstract
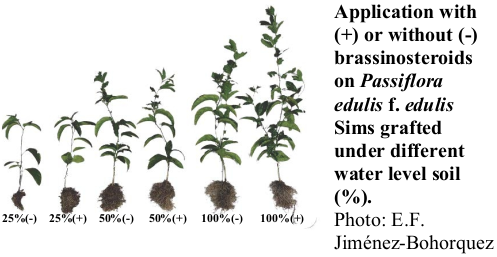
The purple passion fruit is a fruit species of great importance in high Andean areas, but it can be affected by water deficit conditions. The objective of this study was to determine the effect of the application of brassinosteroids on purple passion fruit grafted on Passiflora maliformis and under water deficit. The plants were subjected to different levels of irrigation (100%, 50% and 25% of the evaporated amount) and some were applied with brassinosteroid analogue (DI-31). The fresh and dry mass of the aerial part and the root, leaf area, height of the aerial part, electrolyte loss, relative chlorophyll content, stomatal conductance, and maximum PSII quantum efficiency (Fv/Fm) were determined. It was observed that water stress negatively affected the height, leaf area, and fresh and dry masses of the plants at 84 days after treatment (dat). On the other hand, the results indicate a positive effect of the brassinosteroids on the height, leaf area, and fresh and dry masses of the plants at the different levels of irrigation at 84 dat. An effect of water stress or the exogenous application of brassinosteroids on electrolyte loss was not detected, but these factors did affect Fv/Fm at 28 dat. These results are important for the formulation of integrated management plans for the cultivation of Passiflora edulis f. edulis Sims in a climate change scenario.
Keywords
Passiflora edulis f. edulis Sims, Passiflora maliformis L., Growth, Climate change, Drought, Photosynthesis
References
- Agami, R., S. Alamri, T. Mageed, and M. Abousekken. 2018. Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agric. Water. Manag. 210, 261-270. Doi: https://doi.org/10.1016/j.agwat.2018.08.034
- Ahanger, M.A., N.S. Tomar, M. Tittal, S. Argal, and R. Agarwal. 2017. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants. 23(4), 731-744. Doi: https://doi.org/10.1007/s12298-017-0462-7
- Ahmad Lone, W., N. Majeed, U. Yaqoob, and R. John. 2022. Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Reports. 41(3), 603-617. Doi: https://doi.org/10.1007/s00299-021-02763-9
- Álvarez-Herrera, J., H. González, and G. Fischer. 2019. Water potential in cape gooseberry (Physalis peruviana L.) plants subjected to different irrigation treatments and doses of calcium. Agron. Colomb. 37(3), 274-282. Doi: https://doi.org/10.15446/agron.colomb.v37n3.79935
- Anwar, A., Y. Liu, R. Dong, L. Bai, X. Yu, and Y. Li. 2018. The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol. Res. 51(1), 46. Doi: https://doi.org/10.1186/s40659-018-0195-2
- Barros Junior, U.O., M.D.R. Lima, A.A. Alsahli, and A.K.S. Lobato. 2021. Unraveling the roles of brassinosteroids in alleviating drought stress in young Eucalyptus urophylla plants: Implications on redox homeostasis and photosynthetic apparatus. Physiol Plant. 172(2), 748-761. Doi: https://doi.org/10.1111/ppl.13291
- Behnamnia, M., M. Kalantari, and F. Rezanejad. 2009. Exogenous application of brassinosteroid alleviates drought-induced oxidative stress in Lycopersicon esculentum L. Gen. Appl. Plant Physiol. 35(1), 22-34.
- Castañeda-Murillo, C.C., J.G. Rojas-Ortiz, A.D.S. Sánchez-Reinoso, C.C. Chávez-Arias, and H. Restrepo-Díaz. 2022. Foliar brassinosteroid analogue (DI-31) sprays increase drought tolerance by improving plant growth and photosynthetic efficiency in lulo plants. Heliyon 8(2), e08977. Doi: https://doi.org/10.1016/j.heliyon.2022.e08977
- Chaiwanon, J. and Z.Y. Wang. 2015. Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in arabidopsis roots. Curr. Biol. 25(8), 1031-1042. Doi: https://doi.org/10.1016/j.cub.2015.02.046
- DANE, Departamento Administrativo Nacional de Estadística. 2022. Estadísticas de exportaciones - EXPO - 2011 a 2023. In: www.dane.gov.co; consulted: June, 2023.
- Doležalová, J., M. Koudela, J. Sus, and V. Ptáček. 2016. Effects of synthetic brassinolide on the yield of onion grown at two irrigation levels. Sci. Hortic. 202, 125-132. Doi: https://doi.org/10.1016/j.scienta.2016.02.023
- Eapen, D., M.L. Barroso, G. Ponce, M.E. Campos, and G.I. Cassab. 2005. Hydrotropism: root growth responses to water. Trends Plant Sci. 10(1), 44-50. Doi: https://doi.org/10.1016/j.tplants.2004.11.004
- Fariduddin, Q., M. Yusuf, I. Ahmad, and A. Ahmad. 2014. Brassinosteroids and their role in response of plants to abiotic stresses. Biol. Plant. 58(1), 9-17. Doi: https://doi.org/10.1007/s10535-013-0374-5
- Fischer, G., H.E. Balaguera-López, A. Parra-Coronado, and S. Magnitskiy. 2024. Adaptation of fruit trees to different elevations in the tropical Andes. pp. 193-208. In: Tripathi, S., R. Bhadouria, P. Srivastava, R. Singh, and R.S. Devi (eds.). Ecophysiology of tropical plants - Recent trends and future perspectives. CRC Press, Boca Raton, FL.
- Fischer, G., J.A. Cleves-Leguizamo, and H.E. Balaguera-López. 2022b. Impact of soil temperature on fruit species within climate change scenarios. Rev. Colomb. Cienc. Hortic. 16(1), e12769. Doi: https://doi.org/10.17584/rcch.2022v16i1.12769
- Fischer, G. and D. Miranda. 2021. Review on the ecophysiology of important Andean fruits: Passiflora L. Rev. Fac. Nac. Agron. Medellin 74(2), 9471-9481. Doi: https://doi.org/10.15446/rfnam.v74n2.91828
- Fischer, G., A. Parra-Coronado, and H.E. Balaguera-López. 2022a. Altitude as a determinant of fruit quality with emphasis on the Andean tropics of Colombia. A review. Agron. Colomb. 40(2), 212-227. Doi: https://doi.org/10.15446/agron.colomb.v40n2.101854
- Forero, R., E. Ortiz, W. de León, J.C. Gómez, and L. Hoyos-Carvajal. 2015. Análisis de la resistencia a Fusarium oxysporum en plantas de Passiflora maliformis L. Rev. Colomb. Cienc. Hortic. 9(2), 197-208. Doi: https://doi.org/10.17584/rcch.2015v9i2.4174
- Foyer, C.H. and G. Hanke. 2022. ROS production and signalling in chloroplasts: cornerstones and evolving concepts. Plant J. 111(3), 642-661. Doi: https://doi.org/10.1111/tpj.15856
- Gomes, M.M.A. 2011. Physiological effects related to brassinosteroid application in plants. pp. 193-242 In: Hayat, S. and A. Ahmad (eds). Brassinosteroids: a class of plant hormone. Springer, Dordrecht. Doi: https://doi.org/10.1007/978-94-007-0189-2
- Gomes, M.D.M.A., E. Campostrini, N.R. Leal, A.P. Viana, T.M. Ferraz, L. Nascimento Siqueira, and M.A.T. Zullo. 2006. Brassinosteroid analogue effects on the yield of yellow passion fruit plants (Passiflora edulis f. flavicarpa). Sci. Hortic. 110(3), 235-240. Doi: https://doi.org/10.1016/j.scienta.2006.06.030
- Jiménez, Y., C. Carranza, and M. Rodríguez. 2012. Gulupa (Passiflora edulis Sims.). pp. 579-599. In: Fischer, G. (ed.). Manual para el cultivo de frutales en el trópico. Produmedios, Bogota.
- Khanna-Chopra, R. and D.S. Selote. 2007. Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than-susceptible wheat cultivar under field conditions. Environ. Exp. Bot. 60(2), 276-283. Doi: https://doi.org/10.1016/j.envexpbot.2006.11.004
- Khorobrykh, S., V. Havurinne, H. Mattila, and E. Tyystjärvi. 2020. Oxygen and ROS in photosynthesis. Plants 9(1), 91. Doi: https://doi.org/10.3390/plants9010091
- Kim, Y., Y.S. Chung, E. Lee, P. Tripathi, S. Heo, and K.H. Kim. 2020. Root response to drought stress in rice (Oryza sativa L.). Int. J. Mol. Sci. 21(4), 1513. Doi: https://doi.org/10.3390/ijms21041513
- Kumari, S. and A. Thakur. 2019a. The effects of water stress and brassinosteroid on apple varieties. Int. J. Econ. Plants. 6(1), 1-6. Doi: https://doi.org/10.23910/ijep/2019.6.1.0278
- Kumari, S. and A. Thakur. 2019b. Morphological and physio-biochemical changes in response to foliar application of brassinosteroid and water stress in apple plants under pot culture study. Int. J. Bio-resour. Stress Manag. 10(1), 39-45. Doi: https://doi.org/10.23910/ijbsm/2019.10.1.1944
- Li, X.J., X. Guo, Y.H. Zhou, K. Shi, J. Zhou, J.Q. Yu, and X.J. Xia. 2016. Overexpression of a brassinosteroid biosynthetic gene Dwarf enhances photosynthetic capacity through activation of Calvin cycle enzymes in tomato. BMC Plant Biol. 16(33), 1-12. Doi: https://doi.org/10.1186/s12870-016-0715-6
- Liang, G., J. Liu, J. Zhang, and J. Guo. 2020. Effects of drought stress on photosynthetic and physiological parameters of tomato. J. Am. Soc. Hortic. Sci. 145(1), 12-17. Doi: https://doi.org/10.21273/JASHS04725-19
- Lima, J.V. and A.K.S. Lobato. 2017. Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol. Mol. Biol. Plants. 23(1), 59-72. Doi: https://doi.org/10.1007/s12298-016-0410-y
- Lopez, C., A.H. Salazar, J. Ocampo, D.F.P. Silva, and N.A. Ceballos. 2022. Economic and quality study of purple passion fruit grafted on a fusarium wilt tolerant rootstock. Bragantia 81, e3522. Doi: https://doi.org/10.1590/1678-4499.20220055
- Lozano-Montaña, P.A., F. Sarmiento, L.M. Mejía-Sequera, F. Álvarez-Flórez, and L.M. Melgarejo. 2021. Physiological, biochemical and transcriptional responses of Passiflora edulis Sims f. edulis under progressive drought stress. Sci. Hortic. 275(3), 109655. Doi: https://doi.org/10.1016/j.scienta.2020.109655
- Mahesh, K., P. Balaraju, B. Ramakrishna, and S.S.R. Rao. 2013. Effect of brassinosteroids on germination and seedling growth of radish (Raphanus sativus L.) under PEG-6000 induced water stress. Am. J. Plant Sci. 4(12), 2305-2313. Doi: http://doi.org/10.4236/ajps.2013.412285
- Manghwar, H., A. Hussain, Q. Ali, and F. Liu. 2022. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 23(3), 1012. Doi: https://doi.org/10.3390/ijms23031012
- Molano-Avellaneda, Z., D. Miranda-Lasprilla, and J. Ocampo-Pérez. 2020. Progress in the study of cholupa (Passiflora maliformis L.) phenology in producing areas of Colombia. Rev. Colomb. Cienc. Hortic. 14(1), 32-43. Doi: https://doi.org/10.17584/rcch.2020v14i1.11251
- Nakashima, K., K. Yamaguchi-Shinozaki, and K. Shinozaki. 2014. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 5, 170. Doi: https://doi.org/10.3389/fpls.2014.00170
- Nolan, T.M., N. Vukašinović, D. Liu, E. Russinova, and Y. Yin. 2019. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell. 32(2), 295-318. Doi: https://doi.org/10.1105/tpc.18.00803
- Ocampo, J., A. Rodríguez, and M. Parra. 2020. Gulupa: Passiflora edulis f. edulis Sims. pp. 139-157. In: Rodríguez, A., F.G. Faleiro, M. Parra, and A.M. Costa (eds.). Pasifloras - especies cultivadas en el mundo. ProImpress-Brasilia; Cepass, Brasilia, DF.
- Osorio, J., E. Martínez, J. Clímaco, J. Aguirre, J. Vergara, N. Luque, E. Rojas, and G.N. Cruz. 2020. Caracterización sanitaria de los cultivos de granadilla, gulupa y maracuyá en Colombia, con especial referencia a la secadera causada por Fusarium solani f. sp. passiflorae. Corporación Colombiana de Investigación Agropecuaria – Agrosavia, Mosquera, Colombia.
- Pantin, F., F. Monnet, D. Jannaud, J.M. Costa, J. Renaud, B. Muller, and B. Genty. 2013. The dual effect of abscisic acid on stomata. New Phytol. 197(1), 65-72. Doi: https://doi.org/10.1111/nph.12013
- Paull, R.E. and O. Duarte. 2012. Tropical fruits. 2nd ed. CABI International, Wallingford, UK.
- Peres, A., J. Soares, R. Tavares, G. Righetto, M. Zullo, N. Mandava, and M. Menossi. 2019. Brassinosteroids, the sixth class of phytohormones: a molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 20(2), 331. Doi: https://doi.org/10.3390/ijms20020331
- Pérez-Borroto, L.S., L. Toum, A.P. Castagnaro, J.L. Gonzalez-Olmedo, F. Coll-Manchado, B.G.V. Welin, Y. Coll-García, and E.M. Pardo. 2019. Soybean drought resilience: contributions of a brassinosteroid functional analogue. BioRxiv. 742429. Cold Spring Harbor Laboratory. Doi: https://doi.org/10.1101/742429
- Planas-Riverola, A., A. Gupta, I. Betegón-Putze, N. Bosch, M. Ibañes, and A.I. Caño-Delgado. 2019. Brassinosteroid signaling in plant development and adaptation to stress. Development 146(5), dev151894. Doi: https://doi.org/10.1242/dev.151894
- Ribeiro, D.G.S., B.R.S. Silva, and A.K.S. Lobato. 2019. Brassinosteroids induce tolerance to water deficit in soybean seedlings: contributions linked to root anatomy and antioxidant enzymes. Acta Physiol. Plant. 41(82), 1-11. https://doi.org/10.1007/s11738-019-2873-2
- Rodríguez, A., J. Ocampo, O. Rodríguez, A. Capera, and M. Parra. 2020. Cholupa: Passiflora maliformis L. pp. 123-138. In: Rodríguez, A., F.G. Faleiro, M. Parra, and A.M. Costa (eds.). Pasifloras - especies cultivadas en el mundo. ProImpress-Brasilia; Cepass, Brasilia, DF.
- Schneider, J.R., A. Caverzan, and G. Chavarria. 2019. Water deficit stress, ROS involvement, and plant performance. Arch. Agron. Soil Sci. 65(8), 1160-1181. Doi: https://doi.org/10.1080/03650340.2018.1556789
- Siddiqui, H., S. Hayat, and A. Bajguz. 2018. Regulation of photosynthesis by brassinosteroids in plants. Acta Physiol. Plant. 40(59), 1-15. Doi: https://doi.org/10.1007/s11738-018-2639-2
- Silveira, P.S., J.P.C. Custódio, F.C.M. Silva, A.C.S. Nascente, C.L. Monteiro, and F.S. Matos. 2016. A ação dos brassinosteróides no crescimento de mudas de pinhão manso sob déficit hídrico. Agri-Environ. Sci. 2(1), 52-61. Doi: https://doi.org/10.36725/agries.v2i1.188
- Soma, F., F. Takahashi, K. Yamaguchi-Shinozaki, and K. Shinozaki. 2021. Cellular phosphorylation signaling and gene expression in drought stress responses: ABA-dependent and ABA-independent regulatory systems. Plants. 10(4), 756. Doi: https://doi.org/10.3390/plants10040756
- Taiz, L., E. Zeiger, I.A. Moller, and A. Murphy. 2018. Fundamentals of plant physiology. Sinauer Associates, New York, NY.
- Vieira, D.A., M.A. Toro‐Herrera, A.M.C. Mendonça, and J.P.R.A.D. Barbosa. 2021. Do brassinosteroids alleviate water stress in seedlings of Handroanthus serratifolius?. Ann. Appl. Biol. 180(1), 151-162. Doi: https://doi.org/10.1111/aab.12719
- Wang, X., Y. Gao, Q. Wang, M. Chen, X. Ye, D. Li, and D. Gao. 2019. 24-Epibrassinolide-alleviated drought stress damage influences antioxidant enzymes and autophagy changes in peach (Prunus persica L.) leaves. Plant Physiol. Biochem. 135, 30-40. Doi: https://doi.org/10.1016/j.plaphy.2018.11.026
- Wang, Q., F. Yu, and Q. Xie. 2020. Balancing growth and adaptation to stress: crosstalk between brassinosteroid and abscisic acid signaling. Plant Cell Environ. 43(10), 2325-2335. Doi: https://doi.org/10.1111/pce.13846
- Xia, X.J., L.F. Huang, Y.H. Zhou, W.H. Mao, K. Shi, J.X. Wu, and J.Q. Yu. 2009. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 230(6), 1185-1196. Doi: https://doi.org/10.1007/s00425-009-1016-1
- Yuan, G.F., C.G. Jia, Z. Li, B. Sun, L.P. Zhang, N. Liu, and Q.M. Wang. 2010. Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci. Hortic. 126(2), 103-108. Doi: https://doi.org/10.1016/j.scienta.2010.06.014
