Gnotobiotic system for selecting microorganisms with biocontrol potential against Fusarium oxysporum f. sp. physali
Abstract
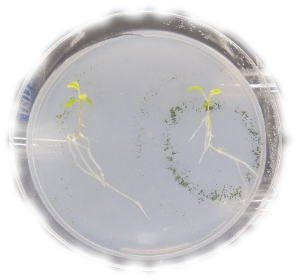
The cape gooseberry (Physalis peruviana) is a Solanaceae species with enormous economic importance in Colombia; it is the second most exported fruit, after bananas. Vascular wilt caused by Fusarium oxysporum f. sp. physali (Fox) is the most limiting factor of this crop, with losses of up to 80% of production. Biological control is a promising alternative for controlling this pathogen. Bacteria and fungi, originally isolated from potentially suppressive soils of cape gooseberry crops in Nariño, Colombia with different management (organic and conventional), were evaluated as biocontrol agents of Fox using a gnotobiotic model (seedlings cultured under axenic conditions with defined microbial strains). Of the 64 isolated microorganisms, 37.5% (15 bacteria and 9 fungi) were discarded because of toxicological risks and an unknow potential biological control. The remaining 62.5% of the microorganisms, 14 bacteria and 26 fungi, were evaluated to assess their potential as biological control agents against Fox. The gnotobiotic model system evaluated the protection and plant growth promotion characteristics. Response variables were used to group the microorganism using a principal component analysis (PCA), and five clusters were obtained. Cluster number four concentrated the 10 microorganisms (three bacteria and seven fungi) with the highest protection values against Fox, with a positive effect on growth. The isolates were identified as two Bacillus subtilis strains, Rhodococcus sp., Podospora setosa, Debaryomyces vindobonensis, Plectosphaerella plurivora, Acinetobacter rhizosphaerae, Umbelopsis sp. and two strains of Trichoderma koningiopsis. The gnobiotic system offered clear advantages for evaluating and selecting microorganisms with a biological control potential against Fusarium oxysporum f. sp. physalis.
Keywords
Biological control, In vitro, Vascular wilt, Rhizosphere, Suppressive soils, Golden berry, Fusarium oxysporum
References
Agronet. 2018. Área, producción y rendimiento nacional por cultivo. In: https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=1; consulted: April, 2019.
Akköprü, A. and S. Demir. 2005. Biological control of Fusarium wilt in tomato caused by Fusarium oxysporum f. sp. lycopersici by AMF Glomus intraradices and some rhizobacteria. J. Phytopathol. 153(9), 544-550. Doi: 10.1111/j.1439-0434.2005.01018.x
Anastasiadis, I.A., I.O. Giannakou, D.A. Prophetou-Athanasiadou, and S.R. Gowen. 2008. The combined effect of the application of a biocontrol agent Paecilomyces lilacinus, with various practices for the control of root-knot nematodes. Crop Prot. 27(3-5), 352-361. Doi: 10.1016/j.cropro.2007.06.008
Arrebola, E., R. Jacobs, and L. Korsten. 2010. Iturin: A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol. 108(2), 386-395. Doi: 10.1111/j.1365-2672.2009.04438.x
Asaka, O. and M. Shoda. 2002. Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl. Environ. Microbiol. 62(11), 4081-4085. Doi: 10.1128/aem.62.11.4081-4085.1996
Bais, H.P., R. Fall, and J.M. Vivanco. 2004. Biocontrol of Bacillus subtilis against infection of arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 134(1), 307-319. Doi: 10.1104/pp.103.028712
Banaay, C.G.B., C.V. Cuevas, and C.M. Vera. 2012. Trichoderma ghanense promotes plant growth and controls disease caused by Pythium arrhenomanes in seedlings of aerobic rice variety Apo. Philipp. Agric. Scientist 95(2), 175-184.
Bayman, P., L.L. Lebron, R.L. Tremblay, and D.J. Lodge. 1997. Variation in endophytic fungi from roots and leaves of Lepanthes (Orchidaceae). New Phytol. 135(1), 143-149. Doi: 10.1046/j.1469-8137.1997.00618.x
Bennett, R.S., W. O’neill, L. Smith, R.B. Hutmacher, and R.S. Bennett. 2011. Plant pathology and nematology: Activity of commercial detergents against conidia and chlamydospores of Fusarium oxysporum f. sp. vasinfectum. J. Cotton Sci. 15(2), 162-169.
Blankenberg, D., A. Gordon, G. Von Kuster, N. Coraor, J. Taylor, A. Nekrutenko, and G. Team. 2010. Manipulation of FASTQ data with galaxy. Bioinformatics 26(14), 1783-1785. Doi: 10.1093/bioinformatics/btq281
Bleve, G., F. Grieco, G. Cozzi, A. Logrieco, and A. Visconti. 2006. Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. Int. J. Food Microbiol. 108(2), 204-209. Doi: 10.1016/j.ijfoodmicro.2005.12.004
Bubici, G., M. Kaushal, M.I. Prigigallo, C.G.L. Cabanás, and J. Mercado-Blanco. 2019. Biological control agents against Fusarium wilt of banana. Front. Microbiol. 10, 616. Doi: 10.3389/fmicb.2019.00616
Caporaso, J.G., C.L. Lauber, W.A. Walters, D. Berg-Lyons, C.A. Lozupone, P.J. Turnbaugh, N. Fierer, and R. Knight. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 108(Suppl. 1), 4516-4522. Doi: 10.1073/pnas.1000080107
Carmarán, C.C. and M.V. Novas. 2003. A review of Spegazzini taxa of Periconia and Sporocybe after over 115 years. Fungal Divers. 14, 67-76.
Carvalho, D.D.C., M. Lobo Junior, I. Martins, P.W. Inglis, and S.C.M. Mello. 2014. Biological control of fusarium oxysporum f. sp. phaseoli by Trichoderma harzianum and its use for common bean seed treatment. Trop. Plant Pathol. 39(5), 384-391. Doi: 10.1590/S1982-56762014000500005
Catara, V. 2007. Pseudomonas corrugata: plant pathogen and/or biological resource? Mol. Plant Pathol. 8(3), 233-244. Doi: 10.1111/j.1364-3703.2007.00391.x
Chastre, J. 2003. Infections due to Acinetobacter baumannii in the ICU. Semin. Respir. Crit. Care Med. 24(1), 069-078. Doi: 10.1055/s-2003-37918
Chen, F., Y.B. Guo, J.H. Wang, J.Y. Li, and H.M. Wang. 2007. Biological control of grape crown gall by Rahnella aquatilis HX2. Plant Dis. 91(8), 957-963. Doi: 10.1094/PDIS-91-8-0957
Chin-A-Woeng, T.F.C., G.V. Bloemberg, and B.J.J. Lugtenberg. 2003. Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol. 157(3), 503-523. Doi: 10.1046/j.1469-8137.2003.00686.x
Cotes, A.M., C.A. Moreno-Velandia, C. Espinel, L. Villamizar, and M. Gómez. 2018. Biological control of tomato Fusarium wilt and whiteflies with two fungal biopesticides. Acta Hortic. 1207, 129-138. Doi: 10.17660/ActaHortic.2018.1207.17
Creason, A.L., O.M. Vandeputte, E.A. Savory, E.W. II Davis, M.L. Putnam, E. Hu, D. Swader-Hines, A. Mol, M. Baucher, E. Prinsen, M. Zdanowska, S.A. Givan, M. El Jaziri, J.E. Loper, T. Mahmud, and J.H. Chang. 2014. Analysis of genome sequences from plant pathogenic Rhodococcus reveals genetic novelties in virulence loci. PLoS ONE 9(7), e101996. Doi: 10.1371/journal.pone.0101996
De Boer Sietske, A. and B. Diderichsen. 1991. On the safety of Bacillus subtilis and B. amyloliquefaciens: a review. Appl. Microbiol. Biotechnol. 36(1), 1-4. Doi: 10.1007/BF00164689
De Hoog, G.S., S.A. Ahmed, M.J. Najafzadeh, D.A. Sutton, M.S. Keisari, A.H. Fahal, U. Eberhardt, G.J. Verkleij, L. Xin, B. Stielow, and W.W.J. van de Sande. 2013. Phylogenetic findings suggest possible new habitat and routes of infection of human eumyctoma. PLoS Negl. Trop. Dis. 7(5), e2229. Doi: 10.1371/journal.pntd.0002229
Deng, W., B.A. Vallance, Y. Li, J.L. Puente, and B.B. Finlay. 2003. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol. Microbiol. 48(1), 95-115. Doi: 10.1046/j.1365-2958.2003.03429.x
Di Pietro, A., M. Gut-Rella, J.P. Pachlatko, and F.J. Schwinn. 1992. Role of Antibiotics Produced by Chaetomium globosum in biocontrol of Pythium ultimum, a causal agent of damping-off. Phytopathology 82(2), 131. Doi: 10.1094/phyto-82-131
Dorman, H.J.D. and S.G. Deans. 2000. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 88(2), 308-316. Doi: 10.1046/j.1365-2672.2000.00969.x
Druzhinina, I.S., V. Seidl-Seiboth, A. Herrera-Estrella, B.A. Horwitz, C.M. Kenerley, E. Monte, P.K. Mukherjee, S. Zeilinger, I.V. Grigoriev, and C.P. Kubicek. 2011. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 9(10), 749-759. Doi: 10.1038/nrmicro2637
El-Tarabily, K.A. 2006. Rhizosphere-competent isolates of streptomycete and non-streptomycete actinomycetes capable of producing cell-wall-degrading enzymes to control Pythium aphanidermatum damping-off disease of cucumber. Can. J. Bot. 84, 211-222. Doi: 10.1139/B05-153
Enciso-Rodríguez, F.E., C. González, E.A. Rodríguez, C.E. López, D. Landsman, L.S. Barrero, and L. Mariño-Ramírez. 2013. Identification of immunity related genes to study the Physalis peruviana - Fusarium oxysporum pathosystem. PLoS ONE 8(7), 68500. Doi: 10.1371/journal.pone.0068500
Eshraghi, L., J.P. Anderson, N. Aryamanesh, J.A. McComb, B. Shearer, and G.E. Giles. 2014. Defence signalling pathways involved in plant resistance and phosphite-mediated control of Phytophthora cinnamomi. Plant Mol. Biol. Rep. 32(2), 342-356. Doi: 10.1007/s11105-013-0645-5
Felestrino, E.B., I.F. Santiago, L.S. Freitas, L.H. Rosa, S.P. Ribeiro, and L.M. Moreira. 2017. Plant growth promoting bacteria associated with Langsdorffia hypogaea-Rhizosphere-Host biological interface: A neglected model of bacterial prospection. Front. Microbiol. 8, 172. Doi: 10.3389/fmicb.2017.00172
Fischer, G. and L.M. Melgarejo. 2020. The ecophysiology of cape gooseberry (Physalis peruviana L.) - an Andean fruit crop. A review. Rev. Colomb. Cienc. Hortic. 14(1), 76-89. Doi: 10.17584/rcch.2020v14i1.10893
Galindo, J.R. and L.M. Pardo. 2010. Uchuva (Physalis peruviana): Producción y manejo poscosecha. Cámara de Comercio, Bogota.
Galindo-Flores, H., J.C. Martínez-Álvarez, E. Nava-Pérez, R.S. García-Estrada, and I.E. Maldonado-Mendoza. 2005. A saprotrophic fungal isolate from northern Sinaloa, Mexico, with homology to members of the chaetomiaceae behaves as an antagonist of phytopathogenic fungi in vitro. Rev. Mex. Fitopatol. 23(2), 130-139.
Gally, T., B.A. González, and F. Pantuso. 2006. Efecto conjunto de Fusarium sp. y Phomopsis sp., patógenos transmitidos por las semillas en plántulas de soja [Glycine max (L.) Merrill]. Rev. Mex. Fitopatol. 24(2), 156-158.
Ghelardi, E., F. Celandroni, S. Salvetti, E. Fiscarelli, and S. Senesi. 2007. Bacillus thuringiensis pulmonary infection: critical role for bacterial membrane-damaging toxins and host neutrophils. Microb. Infect. 9(5), 591-598. Doi: 10.1016/j.micinf.2007.02.001
Giraldo-Betancourt, C., E.A. Velandia-Sánchez, G. Fischer, S. Gómez-Caro, and L.-J. Martínez. 2020. Hyperspectral response of cape gooseberry (Physalis peruviana L.) plants inoculated with Fusarium oxysporum f. sp. physali for vascular wilt detection. Rev. Colomb. Cienc. Hortic. 14(3). Doi: 10.17584/rcch.2020v14i3.10938
González, C. and L.S. Barrero. 2011. Estudio de la marchitez vascular de la uchuva para el mejoramiento genético del cultivo. Cámara de Comercio, Bogota.
Grohskopf, L., V. Roth, D. Feikin, M. Arduino, L. Carson, J. Tokars, S.C. Holt, B.J. Jensen, R.E. Hoffman, and W.R. Jarvis. 2001. Serratia liquefaciens bloodstream infections from contamination of epoetin alfa at a hemodialysis center. N. Engl. J. Med. 344, 1491-1497. Doi: 10.1056/nejm200105173442001
Gunther IV, N.W., A. Nuñez, W. Fett, and D.K.Y. Solaiman. 2005. Production of rhamnolipids by Pseudomonas chlororaphis, a nonpathogenic bacterium. Appl. Environ. Microbiol. 71(5), 2288-2293. Doi: 10.1128/AEM.71.5.2288-2293.2005
Gutiérrez-Luna, F.M., J. López-Bucio, J. Altamirano-Hernández, E. Valencia-Cantero, H.R. De La Cruz, and L. Macías-Rodríguez. 2010. Plant growth-promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis 51(1), 75-83. Doi: 10.1007/s13199-010-0066-2
Gutiérrez, E. 2017. Caracterización de los mecanismos antagónicos de Debaryomyces hansenii contra Colletotrichum gloersporioides y su efecto en la protección poscosecha en papaya var. Maradol. MSc thesis. Centro de Investigaciones Biológicas del Noroeste, La Paz, Mexico.
Haglund, W.A. and J.M. Kraft. 2001. Fusarium wilt. In: Compendium of pea diseases and pests. American Phytopathological Society, St. Paul, MN.
Handelsman, J., S. Raffel, E.H. Mester, L. Wunderlich, and C.R. Grau. 1990. Biological control of damping-off of alfalfa seedlings with Bacillus cereus UW85. Appl. Environ. Microbiol. 56(3), 713-718. Doi: 10.1128/aem.56.3.713-718.1990
Hassanien, M.F.R. 2011. Physalis peruviana: A rich source of bioactive phytochemicals for functional foods and pharmaceuticals. Food Rev. Int. 27(3), 259-273. Doi: 10.1080/87559129.2011.563391
He, H., L.A. Silo-Suh, J. Handelsman, and J. Clardy. 1994. Zwittermicin A: an antifungal and plant protection agent from Bacillus cereus. Tetrahedron Lett. 35(16), 2499-2502. Doi: 10.1016/S0040-4039(00)77154-1
Hoppe, J.E., M. Herter, S. Aleksic, T. Klingebiel, and D. Niethammer. 1993. Catheter-related Rahnella aquatilis bacteremia in a pediatric bone marrow transplant recipient. J. Clin. Microbiol. 31(7), 1911-1912. Doi: 10.1128/JCM.31.7.1911-1912.1993
Howell, C.R. 2003. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 87(1), 4-10. Doi: 10.1094/PDIS.2003.87.1.4
Huang, P., L. de-Bashan, T. Crocker, J.W. Kloepper, and Y. Bashan. 2017. Evidence that fresh weight measurement is imprecise for reporting the effect of plant growth-promoting (rhizo)bacteria on growth promotion of crop plants. Biol. Fert. Soils 53(2), 199-208. Doi: 10.1007/s00374-016-1160-2
Hubbard, J.P., G.E. Harman, and C.J. Eckenrode. 1982. Interaction of a biological control agent, Chaetomium globosum, with seed coat microflora. Can. J. Microbiol. 28(4), 431-437. Doi: 10.1139/m82-065
ICA, Instituto Colombiano Agropecuario. 2020. Fertilizantes y bioinsumos agrícolas. In: https://www.ica.gov.co/getdoc/d3612ebf-a5a6-4702-8d4b-8427c1cdaeb1/registros-nacionales-pqua-15-04-09.aspx; consulted: December, 2020.
Indiragandhi, P., R. Anandham, M. Madhaiyan, and T.M. As. 2008. Characterization of plant growth-promoting traits of bacteria isolated from larval guts of Diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). Curr. Microbiol. 56(4), 327-333. Doi: 10.1007/s00284-007-9086-4
Kalbe, C., P. Marten, and G. Berg. 1996. Strains of the genus Serratia as beneficial rhizobacteria of oilseed rape with antifungal properties. Microbiol. Res. 151(4), 433-439. Doi: 10.1016/S0944-5013(96)80014-0
Kloepper, J.W., F.M. Scher, M. Laliberté, and B. Tipping. 1986. Emergence-promoting rhizobacteria: Description and implications for agriculture. pp. 155-164. In: Swinburne, T.R. (eds.). Iron, siderophores, and plant diseases. NATO ASI Series (Series A: Life Sciences). Vol. 117. Springer, Boston, MA. Doi: 10.1007/978-1-4615-9480-2_17
Köhl, J., R. Kolnaar, and W.J. Ravensberg. 2019. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 10, 845. Doi: 10.3389/fpls.2019.00845
Korolev, N., J. Katan, and T. Katan. 2000. Vegetative compatibility groups of Verticillium dahliae in Israel: Their distribution and association with pathogenicity. Phytopathology 90(5), 529-536. Doi: 10.1094/PHYTO.2000.90.5.529
Kůdela, V., V. Krejzar, and I. Pánková. 2010. Pseudomonas corrugata and Pseudomonas marginalis associated with the collapse of tomato plants in Rockwool slab hydroponic culture. Plant Prot. Sci. 46(1), 1-11. Doi: 10.17221/44/2009-PPS
Kuhls, K., E. Lieckfeldt, T. Börner, and E. Guého. 1999. Molecular reidentification of human pathogenic Trichoderma isolates as Trichoderma longibrachiatum and Trichoderma citrinoviride. Med. Mycol. 37(1), 25-33. Doi: 10.1046/j.1365-280X.1999.00197.x
Kumar, R.M., G. Kaur, A. Kumar, M. Bala, N.K. Singh, N. Kaur, N. Kumar, and S. Mayilraj. 2015. Taxonomic description and genome sequence of Bacillus campisalis sp. nov., a member of the genus Bacillus isolated from a solar saltern. Int. J. Syst. Evol. Microbiol. 65(10), 3235-3240. Doi: 10.1099/ijsem.0.000400
Kurze, S., H. Bahl, R. Dahl, and G. Berg. 2001. Biological control of fungal strawberry diseases by Serratia plymuthica HRO-C48. Plant Dis. 85(5), 529-534. Doi: 10.1094/PDIS.2001.85.5.529
Lagier, J.C., F. Armougom, M. Million, P. Hugon, I. Pagnier, C. Robert, F. Bittar, G. Fournous, G. Gimenez, M. Maraninchi, J.-F. Trape, E.V. Koonin, B. La Scola, and D. Raoult. 2012. Microbial culturomics: Paradigm shift in the human gut microbiome study. Clin. Microbiol. Infect. 18(12), 1185-1193. Doi: 10.1111/1469-0691.12023
López, J.R., A.L. Diéguez, A. Doce, E. de la Roca, R. de la Herran, J.I. Navas, A.E. Toranzo, and J.L. Romalde. 2012. Pseudomonas baetica sp. nov., a fish pathogen isolated from wedge sole, Dicologlossa cuneata (Moreau). Int. J. Syst. Evol. Microb. 62(4), 874-882. Doi: 10.1099/ijs.0.030601-0
Lorito, M., S.L. Wood, M. D’Ambrosio, G.E. Harman, C.K. Hayes, C.P. Kubicek, and F. Scala. 1996. Synergistic interaction between cell wall degrading enzymes and membrane affecting compounds. Mol. Plant Microbe Interact. 9(3), 206-213. Doi: 10.1094/MPMI-9-0206
Lucy, M., E. Reed, and B.R. Glick. 2004. Applications of free living plant growth-promoting rhizobacteria. Antonie van Leeuwenhoek 86(1), 1-25. Doi: 10.1023/B:ANTO.0000024903.10757.6e
Luongo, L., M. Galli, L. Corazza, E. Meekes, L. De Haas, C.L. Van Der Plas, and J. Köhl. 2005. Potential of fungal antagonists for biocontrol of Fusarium spp. in wheat and maize through competition in crop debris. Biocontrol Sci. Techn. 15(3), 229-242. Doi: 10.1080/09583150400016852
Marinho, A.M.R., E. Rodrigues-Filho, M.D.L.R. Moitinho, and L.S. Santos. 2005. Biologically active polyketides produced by Penicillium janthinellum isolated as an endophytic fungus from fruits of Melia azedarach. J. Braz. Chem. Soc. 16(2), 280-283. Doi: 10.1590/s0103-50532005000200023
Martínez, S.A. and J. Dussán. 2018. Lysinibacillus sphaericus plant growth promoter bacteria and lead phytoremediation enhancer with Canavalia ensiformis. Environ. Prog. Sustain. Energy 37(1), 276-282. Doi: 10.1002/ep.12668
Martínez-Medina, A., M. Del Mar Alguacil, J.A. Pascual, and S.C.M. Van Wees. 2014. Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J. Chem. Ecol. 40(7), 804-815. Doi: 10.1007/s10886-014-0478-1
Mayorga-Cubillos, F., J. Arguelles, E. Rodriguez, C. Almario, C. Ariza, and L. Barrero. 2019. Yield and physicochemical quality of Physalis peruviana L. fruit related to the resistance response against Fusarium oxysporum f. sp. physali. Agron. Colomb. 37(2), 120-128. Doi: 10.15446/agron.colomb.v37n2.77550
McGovern, R.J. 2015. Management of tomato diseases caused by Fusarium oxysporum. Crop Prot. 73, 78-92. Doi: 10.1016/j.cropro.2015.02.021
Melnick, R.L., C. Suárez, B.A. Bailey, and P.A. Backman. 2011. Isolation of endophytic endospore-forming bacteria from Theobroma cacao as potential biological control agents of cacao diseases. Biol. Control 57(3), 236-245. Doi: 10.1016/j.biocontrol.2011.03.005
Michielse, C.B. and M. Rep. 2009. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 10(3), 311-324. Doi: 10.1111/j.1364-3703.2009.00538.x
Moreno, C.A., F. Castillo, A. González, D. Bernal, Y. Jaimes, M. Chaparro, C. González, F. Rodriguez, S. Restrepo, and A.M. Cotes. 2009. Biological and molecular characterization of the response of tomato plants treated with Trichoderma koningiopsis. Physiol. Mol. Plant Pathol. 74(2), 111-120. Doi: 10.1016/j.pmpp.2009.10.001
Moreno, C.A., J. Kloepper, M. Ongena, and A.M. Cotes. 2014. Biotic factors involved in biological control activity of Bacillus amyloliquefaciens (Bs006) against Fusarium oxysporum in cape gooseberry (Physalis peruviana). pp. 129-136. In: Pertot, I., D.F. Jensen, M. Hökeberg, M. Karlsson, I. Sundh, and Y. Elad (eds.). Proc. XIII Meeting Biocontrol of Plant Diseases: From the Field to the Laboratory and Back Again. IOBC-WPRS Bull. 115. Zürich Switzerland.
Moreno-Limón, S., L.N. González-Solís, S.M. Salcedo-Martínez, M.L. Cárdenas-Avila, and A. Perales-Ramírez. 2011. Efecto antifúngico de extractos de gobernadora (Larrea tridentata L.) sobre la inhibición in vitro de Aspergillus flavus y Penicillium sp. Polibotánica 32, 193-205.
NRCS, National Resources Conservation Service. 2020. Physalis peruviana L.: Peruvian groundcherry. In: United States Department of Agriculture USDA, https://plants.sc.egov.usda.gov/core/profile?symbol=PHPE4; consulted: November, 2020.
Nicoletti, R., A. Carella, and E. Cozzolino. 2008. Investigation on fungal antagonists of root rot agents from the rhizosphere of white lupin (Lupinus albus). Dyn. Soil Dyn. Plant 2(1), 69-72.
Opelt, K., V. Chobot, F. Hadacek, S. Schönmann, L. Eberl, and G. Berg. 2007. Investigations of the structure and function of bacterial communities associated with Sphagnum mosses. Environ. Microbiol. 9(11), 2795-2809. Doi: 10.1111/j.1462-2920.2007.01391.x
Osburn, R.M., J.L. Milner, E.S. Oplinger, R.S. Smith, and J. Handelsman. 1995. Effect of Bacillus cereus UW85 on the yield of soybean at two field sites in Wisconsin. Plant Dis. 79(6), 551-556. Doi: 10.1094/PD-79-0551
Ownley, B.H., M.R. Griffin, W.E. Klingeman, K.D. Gwinn, J.K. Moulton, and R.M. Pereira. 2008. Beauveria bassiana: Endophytic colonization and plant disease control. J. Invertebr. Pathol. 98(3), 267-270. Doi: 10.1016/j.jip.2008.01.010
Palma-Guerrero, J., H.B. Jansson, J. Salinas, and L.V. Lopez-Llorca. 2008. Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. J. Appl. Microbiol. 104(2), 541-553. Doi: 10.1111/j.1365-2672.2007.03567.x
Pieterse, C.M.J., C. Zamioudis, R.L. Berendsen, D.M. Weller, S.C.M. Van Wees, and P.A.H.M. Bakker. 2014. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347-375. Doi: 10.1146/annurev-phyto-082712-102340
Ploper, L.D., P.A. Backman, C. Stevens, V.A. Khan, and R. Rodriguez-Kábana. 1992. Effects of soil mulch, row cover, and biological and chemical foliar treatments on early blight of tomato. Biol. Cult. Test. 7, 38.
Prescott, J.F. 1991. Rhodococcus equi: An animal and human pathogen. Clin. Microbiol. Rev. 4(1), 20-34. Doi: 10.1128/CMR.4.1.20
Raaijmakers, J.M. and D.M. Weller. 1998. Natural plant protection by 2,4-diacetylphloroglucinol-producing Pseudomonas spp. in Take-all decline soils. Mol. Plant-Microb. Interact. 11(2), 144-152. Doi: 10.1094/MPMI.1998.11.2.144
Rai, J.N. and V.C. Saxena. 1975. Sclerotial mycoflora and its role in natural biological control of ‘white-rot’ disease. Plant Soil 43(1), 509-513. Doi: 10.1007/BF01928513
Rashid, S., T.C. Charles, and B.R. Glick. 2012. Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl. Soil Ecol. 61, 217-224. Doi: 10.1016/j.apsoil.2011.09.011
Refai, M., H.A. El-Yazid, and W. Tawakkol. 2015. Monograph on the genus Penicillium. A guide for historical, classification and identification of penicilli, their industrial applications and detrimental effects. Cairo University, Cairo.
Rete, A. 2011. Caracterización molecular de Streptomyces spp. asociadas a la sarna común y evaluación in vitro de antagonistas nativos de la rizosfera de papa en Sinaloa. MSc thesis. Instituto Politécnico Nacional, Guasave, Mexico.
Rickerts, V., A. Böhme, A. Viertel, G. Behrendt, V. Jacobi, K. Tintelnot, and G. Just-Nübling. 2000. Cluster of pulmonary infections caused by Cunninghamella bertholletiae in immunocompromised patients. Clin. Infect. Dis. 31(4), 910-913. Doi: 10.1086/318144
Rodriguez, E. 2010. Aislamiento y caracterización de cepas de Fusarium oxysporum en uchuva (Physalis peruviana) y evaluación de la patogenicidad en invernadero. Undergraduate thesis. Universidad de Cundinamaca, Fusagasuga, Colombia.
Rodríguez, E.A. 2013. Caracterización de aislamientos de Fusarium spp. obtenidos de zonas productoras de uchuva (Physalis peruviana) en Cundinamarca y Boyacá. MSc tesis. Facultad de Agronomía, Universidad Nacional de Colombia. Bogota.
Rodríguez, P., A. Cerda, X. Font, A. Sánchez, and A. Artola. 2019. Valorisation of biowaste digestate through solid state fermentation to produce biopesticides from Bacillus thuringiensis. Waste Manage. 93, 63-71. Doi: 10.1016/j.wasman.2019.05.026
Rokhbakhsh-Zamin, F., D. Sachdev, N. Kazemi-Pour, A. Engineer, K.R. Pardesi, S. Zinjarde, P.K. Dhakephalkar, and B.A. Chopade. 2011. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol. 21(6), 556-566. Doi: 10.4014/jmb.1012.12006
Sahebani, N. and N. Hadavi. 2008. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biol. Biochem. 40(8), 2016-2020. Doi: 10.1016/j.soilbio.2008.03.011
Sandoval-Denis, M., J. Guarro, J.F. Cano-Lira, D.A. Sutton, N.P. Wiederhold, G.S. de Hoog, S.P. Abbott, C. Decock, L. Sigler, and J. Gené. 2016. Phylogeny and taxonomic revision of Microascaceae with emphasis on synnematous fungi. Stud. Mycol. 83, 193-233. Doi: 10.1016/j.simyco.2016.07.002
Schirmböck, M., M. Lorito, Y.L. Wang, C.K. Hayes, I. Arisan-Atac, F. Scala, G.E. Harman, and C.P. Kubicek. 1994. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl. Environ. Microbiol. 60(12), 4364-4370. Doi: 10.1128/aem.60.12.4364-4370.1994
Shishido, M., C. Miwa, T. Usami, Y. Amemiya, and K.B. Johnson. 2005. Biological control efficiency of Fusarium wilt of tomato by nonpathogenic Fusarium oxysporum Fo-B2 in different environments. Phytopathology 95(9), 1072-1080. Doi: 10.1094/PHYTO-95-1072
Shoda, M. 2000. Bacterial control of plant diseases. J. Biosci. Bioeng. 89(6), 515-521. Doi: 10.1016/S1389-1723(00)80049-3
Siegel-Hertz, K., V. Edel-Hermann, E. Chapelle, S. Terrat, J.M. Raaijmakers, and C. Steinberg. 2018. Comparative microbiome analysis of a Fusarium wilt suppressive soil and a Fusarium wilt conducive soil from the Châteaurenard region. Front. Microbiol. 9, 568. Doi: 10.3389/fmicb.2018.00568
Singh, R.K., D.P. Kumar, P. Singh, M.K. Solanki, S. Srivastava, P.L. Kashyap, S. Kumar, A.K. Srivastava, P.K. Singhal, and D.K. Arora. 2014. Multifarious plant growth promoting characteristics of chickpea rhizosphere associated Bacilli help to suppress soil-borne pathogens. Plant Growth Regulation, 73(1), 91-101. Doi: 10.1007/s10725-013-9870-z
Singh, N.P., R.K. Singh, V.S. Meena, and R.K. Meena. 2015. Can we use maize (Zea mays) rhizobacteria as plant growth promoter? Vegetos 28(1), 86-99. Doi: 10.5958/2229-4473.2015.00012.9
Smith, A. 2012. Reconocimiento de las enfermedades y plagas en el cultivo de uchuva. pp. 9-12. In: Días, A. (ed.). Avances en el manejo y control de Fusarium oxysporum en el cultivo de uchuva (Physalis peruviana). Corpoica, Bogota, DC.
Sobita, S. and A. Anamika. 2011. Agro-based waste products as a substrate for mass production of Trichoderma spp. J. Agric. Sci. 3(4), 168-171. 10.5539/jas.v3n4p168
Spencer, R.C. 2003. Bacillus anthracis. J. Clin. Pathol. 56(3), 182-187. Doi: 10.1136/jcp.56.3.182
Szaniszlo, P.J. and J.L. Harris (eds.). 1985. Fungal dimorphism: With emphasis on fungi pathogenic for humans. Springer, Boston, MA. Doi: 10.1007/978-1-4684-4982-2
Tena, D., C. Fernández, and M.R. Lago. 2015. Alcaligenes faecalis: An unusual cause of skin and soft tissue infection. Jap. J. Infect. Dis. 68(2), 128-130. Doi: 10.7883/yoken.JJID.2014.164
Toju, H., A.S. Tanabe, S. Yamamoto, and H. Sato. 2012. High-coverage ITS primers for the DNA-based identification of Ascomycetes and Basidiomycetes in environmental samples. PLoS ONE 7(7), e40863. Doi: 10.1371/journal.pone.0040863
Toloza-Moreno, D.L., L.M. Lizarazo-Forero, and D. Uribe-Vélez. 2020. Antagonist capacity of bacteria isolated from cape gooseberry cultures (Physalis peruviana L.) for biological control of Fusarium oxysporum. Trop. Plant Pathol. 45(1), 1-12. Doi: 10.1007/s40858-019-00313-z
Urrea, R., L. Cabezas, R. Sierra, M. Cárdenas, S. Restrepo, and P. Jiménez. 2011. Selection of antagonistic bacteria isolated from the Physalis peruviana rhizosphere against Fusarium oxysporum. J. Appl. Microbiol. 111(3), 707-716. Doi: 10.1111/j.1365-2672.2011.05092.x
Velivelli, S.L.S., P. Kromann, P. Lojan, M. Rojas, J. Franco, J.P. Suarez, and B.D. Prestwich. 2015. Identification of mVOCs from andean rhizobacteria and field evaluation of bacterial and mycorrhizal inoculants on growth of potato in its center of origin. Microbiol. Ecol. 69(3), 652-667. Doi: 10.1007/s00248-014-0514-2
Verma, P.S. and C.C. Allison. 1970. Possible modification of susceptibility of tomato to Fusarium wilt by a Chaetomium sp. Phytopathology 60, 1318.
Villarreal-Navarrete, A., G. Fischer, L.M. Melgarejo, G. Correa, and L. Hoyos-Carvajal. 2017. Growth response of the cape gooseberry (Physalis peruviana L.) to waterlogging stress and Fusarium oxysporum infection. Acta Hortic. 1178, 161-168. Doi: 10.17660/ActaHortic.2017.1178.28
Von Der Weid, I., D.S. Alviano, A.L.S. Santos, R.M.A. Soares, C.S. Alviano, and L. Seldin. 2003. Antimicrobial activity of Paenibacillus peoriae strain NRRL BD-62 against a broad spectrum of phytopathogenic bacteria and fungi. J. Appl. Microbiol. 95(5), 1143-1151. Doi: 10.1046/j.1365-2672.2003.02097.x
Xu, L., S. Ravnskov, J. Larsen, R.H. Nilsson, and M. Nicolaisen. 2012. Soil fungal community structure along a soil health gradient in pea fields examined using deep amplicon sequencing. Soil Biol. Biochem. 46, 26-32. Doi: 10.1016/j.soilbio.2011.11.010
Zacky, F.A. and A.S.Y. Ting. 2013. Investigating the bioactivity of cells and cell-free extracts of Streptomyces griseus towards Fusarium oxysporum f. sp. cubense race 4. Biol. Control 66(3), 204-208. Doi: 10.1016/j.biocontrol.2013.06.001
Zahid, M., M. Kaleem Abbasi, S. Hameed, and N. Rahim. 2015. Isolation and identification of indigenous plant growth promoting rhizobacteria from Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.). Front. Microbiol. 6, 207. Doi: 10.3389/fmicb.2015.00207
Zhang, X., P.R. Harvey, B.E. Stummer, R.A. Warren, G. Zhang, K. Guo, J. Li, and H. Yang. 2015. Antibiosis functions during interactions of Trichoderma afroharzianum and Trichoderma gamsii with plant pathogenic Rhizoctonia and Pythium. Funct. Integr. Genomics 15, 599-610. Doi: 10.1007/s10142-015-0456-x
Zhao, S., D. Liu, N. Ling, F. Chen, W. Fang, and Q. Shen. 2014. Bio-organic fertilizer application significantly reduces the Fusarium oxysporum population and alters the composition of fungi communities of watermelon Fusarium wilt rhizosphere soil. Biol. Fertil. Soils 50(5), 765-774. Doi: 10.1007/s00374-014-0898-7
