Spontaneous and induced microbial consortia: the key to high-quality cocoa
Abstract
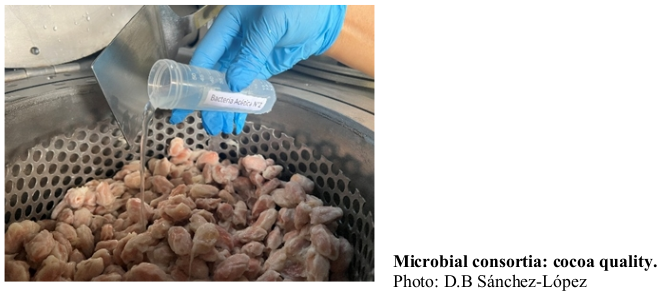
Cocoa fermentation enables the activity of microorganisms that are crucial for its aroma and flavor. This study evaluated how to improve the post-harvest quality of cocoa using specific microbial consortia and a controlled fermentation device at six different time points: 0, 24, 48, 72, 96, and 120 hours. Three treatments were compared using CCN51 cocoa beans: spontaneous fermentation (FESP); inoculation with Saccharomyces cerevisiae-CAO-004, Issatchenkia orientalis-CAO-003, and Gluconacetobacter sp.-CAO-008 (SIGO); and inoculation with Saccharomyces cerevisiae-CAO-004, Lactobacillus plantarum-CAO-009, and Acetobacter pasteurianus-CAO-010 (SILA). Analyses included pH, microbial dynamics, organic acids, alkaloids, polyphenols, antioxidant capacity, and sensory profile. Treatments involving the inoculation of microbial consortia not only increased the populations of yeasts, lactic acid bacteria, and acetic acid bacteria, but also altered the levels of organic acids and significantly enhanced quality. These treatments classified the cocoa as premium and showed significant differences in compounds such as caffeine and flavonoids like catechins. The SIGO treatment achieved the highest score (8.0) due to its balance of flavors and fruity, sweet, and nutty notes. SILA, with a score of 7.5, also stood out for its flavor balance and fruity, nutty, and floral attributes. The inoculation of specific microbial consortia and the use of a controlled fermentation device improve the quality and flavor of CCN51 cocoa, offering a valuable tool to optimize fermentation and enhance the final product ultimately improving cocoa quality and competitiveness while fostering innovation and sustainability in the industry, optimizing the process to ensure a high-value final product.
Keywords
Cocoa quality, Genetic material, Fermentation, Theobroma cacao
Supplementary File(s)
PDF Suppl 2References
- Acevedo-Osorio, Á., J.S. Santoyo-Sánchez, P. Guzmán, and N. Jiménez-Reinales. 2018. Family farming facing the extractive model of rural development in Colombia. Gest. Ambient. 21(Suppl. 2), 144. Doi: https://doi.org/10.15446/ga.v21n2supl.73925
- Acierno, V. 2020. Following cocoa beans to chocolate: The search for intrinsic characteristics. PhD thesis, University Wageningen, Wageningen, The Netherlands.
- Afoakwa, E.O., J. Quao, J. Takrama, A.S. Budu, and F.K. Saalia. 2013. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J. Food Sci. Technol. 50(6), 1097-1105. Doi: https://doi.org/10.1007/s13197-011-0446-5
- Aria, P.S. (ed.). 2023. AOAC official method 970.21. pH of cacao products: potentiometric method. In: Latimer, G.W. (ed.). Official methods of analysis of AOAC international. 22th ed. New York, NY. Doi: https://doi.org/10.1093/9780197610145.003.2884
- Barišić, V., N.C. Icyer, S. Akyil, O.S. Toker, I. Flanjak, and Đ. Ačkar. 2023. Cocoa based beverages–Composition, nutritional value, processing, quality problems and new perspectives. Trends Food Sci. Technol. 132, 65-75. Doi: https://doi.org/10.1016/j.tifs.2022.12.011
- Benzie, I.F.F. and J.J. Strain. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239(1), 70-76. Doi: http://dx.doi.org/10.1006/abio.1996.0292
- Bhat, R., B. Bhavishya, and S. Sujatha. 2024. Cocoa (Theobroma cacao L.). In: Thomas, G.V. and V. Krishnakumar (eds.). Soil health management for plantation crops. Springer, Singapore. Doi: https://doi.org/10.1007/978-981-97-0092-9_8
- Camu, N., T. De Winter, K. Verbrugghe, I. Cleenwerck, P. Vandamme, J.S. Takrama, M. Vancanneyt, and L. De Vuyst. 2007. Dynamics and biodiversity of populations of lactic acid bacteria and acetic acid bacteria involved in spontaneous heap fermentation of cocoa beans in Ghana. Appl. Environ. Microbiol. 73, 1809-1824. Doi: https://doi.org/10.1128/AEM.02189-06
- Castillejos-Mijangos, L.A., O.G. Meza-Márquez, G. Osorio-Revilla, C. Jiménez-Martínez, and T. Gallardo-Velázquez. 2023. Identification of variety and prediction of chemical composition in cocoa beans (Theobroma cacao L.) by FT-MIR spectroscopy and chemometrics. Foods 12(22), 4144. Doi: https://doi.org/10.3390/foods12224144
- Cevallos-Cevallos, J.M., L. Gysel, M.G. Maridueña-Zavala, and M.J. Molina-Miranda. 2018. Time‐related changes in volatile compounds during fermentation of bulk and fine‐flavor cocoa (Theobroma cacao) beans. J. Food Qual. 2018(1), 1758381. Doi: https://doi.org/10.1155/2018/1758381
- Clark, D.H. (ed.). 2023. AOAC official method 942.15 acidity (titratable) of fruit products. C37-11. In: Latimer, G.W. (ed.). Official methods of analysis of AOAC International. 22th ed. New York, NY. Doi: https://doi.org/10.1093/9780197610145.003.3390
- De Vuyst, L. and F. Leroy. 2020. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 44(4), 432-453. Doi: https://doi.org/10.1093/femsre/fuaa014
- De Vuyst, L. and S. Weckx. 2016. The cocoa bean fermentation process: from ecosystem analysis to starter culture development. J. Appl. Microbiol. 121(1), 5-17. Doi: https://doi.org/10.1111/jam.13045
- Falconí, C.E., V. Yánez-Mendizábal, R.J. Haro, and D.R. Claudio. 2023. Inoculum of a native microbial starter cocktail to optimize fine-aroma cocoa (Theobroma cacao) bean fermentation. Agronomy 13(10), 2572. Doi: https://doi.org/10.3390/agronomy13102572
- Ganeswari, I., K.S. Bariah, M.A. Amizi, and K.Y. Sim. 2015. Effects of different fermentation approaches on the microbiological and physicochemical changes during cocoa bean fermentation. Int. Food Res. J. 22(1), 70-76.
- Hirko, B., H. Mitiku, and A. Getu. 2023. Role of fermentation and microbes in cacao fermentation and their impact on cacao quality. Syst. Microbiol. Biomanufacturing 3(4), 509-520. Doi: https://doi.org/10.1007/s43393-023-00160-9
- ICONTEC, Instituto Colombiano de Normas Técnicas y Certificación. 2004. NTC 5278. Análisis Sensorial. Metodología. Análisis secuencial. Bogota.
- ICONTEC, Instituto Colombiano de Normas Técnicas y Certificación. 2009. NTC 3929. Análisis sensorial. Metodología. Métodos del perfil del sabor. Bogota.
- ICONTEC, Instituto Colombiano de Normas Técnicas y Certificación. 2012. NTC 3501. Análisis Sensorial. Vocabulario. Bogota.
- ICONTEC, Instituto Colombiano de Normas Técnicas y Certificación. 2017. GTC 280. Análisis sensorial. Directrices para la selección, entrenamiento y seguimiento de evaluadores sensoriales seleccionados y expertos. Bogota.
- ICONTEC, Instituto Colombiano de Normas Técnicas y Certificación. 2020. GTC 232. Análisis sensorial. Metodología. Guía general para el establecimiento de un perfil sensorial. Bogota.
- ICONTEC, Instituto Colombiano de Normas Técnicas y Certificación. 2021 NTC 1252. Cacao en grano. especificaciones y requisitos de calidad. Bogota.
- Kadow, D., N. Niemenak, S. Rohn, and R. Lieberei. 2015. Fermentation-like incubation of cocoa seeds (Theobroma cacao L.) Reconstruction and guidance of the fermentation process. LWT Food Sci Technol. 62(1), 357-361. Doi: https://doi.org/10.1016/j.lwt.2015.01.015
- Lagos-Quispe, T.M., E.E. Vásquez-Montenegro, G. Rojas-Yauri, I.L. Huamani-Urpe, and J.C. Sosa-Choque. 2024. Fermentación de cacao criollo y CCN-51: bacteria Lactobacillus fermentum y levadura Saccharomyces cerevisiae. Rev. Univ. Soc. 16(4), 52-63.
- Li, Y., Y. Feng, S. Zhu, C. Luo, J. Ma, and F. Zhong. 2012. The effect of alkalization on the bioactive and flavor related components in commercial cocoa powder. J. Food Compost. Anal. 25(1), 17-23. Doi: https://doi.org/10.1016/j.jfca.2011.04.010
- MinAgricultura, Ministerio de Agricultura y Desarrollo Rural. 2023. Agronet: Cifras agropecuarias. Agricola Cacao https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=1; consulted: April, 2025.
- Mota-Gutierrez, J., C. Botta, I. Ferrocino, M. Giordano, M. Bertolino, P. Dolci, M. Cannoni, and L. Cocolin. 2018. Dynamics and biodiversity of bacterial and yeast communities during fermentation of cocoa beans. Appl. Environ. Microbiol. 84(19), e01164-18. Doi: https://doi.org/10.1128/AEM.01164-18
- Navajas-Porras, B., S. Pérez-Burillo, J. Morales-Pérez, J.A. Rufián-Henares, and S. Pastoriza. 2020. Relationship of quality parameters, antioxidant capacity and total phenolic content of EVOO with ripening state and olive variety. Food Chem. 325, 126926. Doi: https://doi.org/10.1016/j.foodchem.2020.126926
- Nielsen, D.S., O.D. Teniola, L. Ban-Koffi, M. Owusu, T.S. Andersson, and W.H. Holzapfel. 2007. The microbiology of Ghanaian cocoa fermentations analysed using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 114(2), 168-186. Doi: https://doi.org/10.1016/j.ijfoodmicro.2006.09.010
- Ordoñez, E.S., Y. Quispe, and L.F. García. 2020. Quantification of phenols, anthocyanins and sensory characterization of nibs and liquor of five cocoa varieties, in two fermentation systems. Sci. Agropecu. 11(4), 473-481. Doi: https://doi.org/10.17268/sci.agropecu.2020.04.02
- Pallares, A.P., M. Estupiñán, J.A.P. Villamil, and L.J.L. Giraldo. 2016. Impacto de la fermentación y secado sobre el contenido de polifenoles y capacidad antioxidante del clon de cacao CCN-51. Rev. Ion. 29, 7-21. Doi: https://doi.org/10.18273/revion.v29n2-2016001
- Quintana, L. and A. García. 2021. Evaluación integral de la calidad sensorial del cacao. UNAD, Bogota. Doi: https://doi.org/10.22490/9789586517782
- Ramírez, L., E. Chávez, D. Bravo, B. Moyano, and V. Sánchez. 2024. Plataforma multiagencia de cacao. ATN/RF-17235-RG. In: https://www.fontagro.org/new/uploads/productos/17235_-_Producto_12.pdf; consulted: April, 2025.
- Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26(9-10), 1231-1237. Doi: https://doi.org/10.1016/S0891-5849(98)00315-3
- Sánchez-López, D.B., L.G. Rodriguez-Silva, M.R. Espinosa-Carvajal, and R.A. Coronado-Silva. 2023. Microbiological, sensory and bromatological characterization of cocoa fermentation in ICS 95 and CCN 51 genotypes FAVE. Secc. Cienc. Agrar. 22, e0016. Doi: https://doi.org/10.14409/fa.2023.22.e0016
- Toapanta, M.G.C., G.K.M. Pilay, G.T.S. Paula, and H.A.M. Cano. 2023. Métodos de fermentación en el cacao CCN-51 con norma INEN 176 en la parroquia Guasaganda. Polo del Conocimiento: Rev. Científ. Prof. 8: 613-633. Doi: https://doi.org/10.23857/pc.v8i6
- Van de Voorde, D., C. Díaz-Muñoz, C.E. Hernandez, S. Weckx, and L. De Vuyst. 2023. Yeast strains do have an impact on the production of cured cocoa beans, as assessed with Costa Rican Trinitario cocoa fermentation processes and chocolates thereof. Front. Microbiol. 14, 1232323. Doi: https://doi.org/10.3389/fmicb.2023.1232323
- Viesser, J.A., G.V.M. Pereira, D.P. Carvalho Neto, H. Rogez, A. Góes-Neto, V. Azevedo, B. Brenig, F. Aburjaile, and C.R. Soccol. 2021. Co-culturing fructophilic lactic acid bacteria and yeast enhanced sugar metabolism and aroma formation during cocoa beans fermentation. Int. J. Food Microbiol. 339, 109015. Doi: https://doi.org/10.1016/j.ijfoodmicro.2020.109015
- Wills, C. 2008. Regulation of sugar and ethanol metabolism in Saccharomyces cerevisiae. Crit. Rev. Biochem. Mol. Biol. 25(4), 245-280. Doi: https://doi.org/10.3109/10409239009090611
- Zhang, W., P. Weng, and Z. Wu. 2019. Fermentation efficiency and flavor characteristics of bayberry wine with mixed starter culture of Issatchenkio orientalis and Saccharomyces cerevisiae, Shipin Kexue. Food Sci. 40(18), 144-151. Doi: https://doi.org/10.7506/spkx1002-6630-20180831-375
