Molecular detection of three RNA viruses in tomato (Solanum lycopersicum L.) leaf tissue and seeds in Antioquia (Colombia)
Abstract
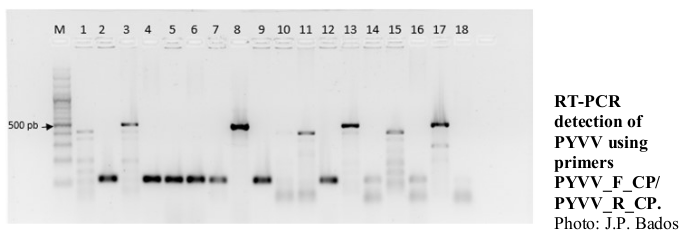
Viruses are a significant threat to tomato crops (Solanum lycopersicum) worldwide. In Colombia, 20 species of viruses from 10 genera have been reported in tomato, with 9 of them being RNA viruses that cause varying levels of damage. This study in Antioquia evaluated the presence of three RNA viruses: potato virus Y (PVY), potato yellow vein virus (PYVV) and Southern tomato virus (STV) in different tomato seeds and plants using RT-qPCR, conventional RT-PCR and Sanger sequencing. The study included 15 leaf tissue samples of the Chonto variety and 15 seed samples of various tomato varieties (Cherry, Chonto, Yellow, Kidney, San Marzano, and Mexican husk tomato). The results showed high levels of PVY in leaf tissue (93.3%) and seeds (46.6%), while PYVV was detected in 60% of leaf samples and 26.6% of seed samples. STV was found in 93.3% of seeds but was not detected in any leaf samples. Sanger sequencing confirmed the presence of these viruses, and phylogenetic analyses placed the isolates in clades previously reported for these viruses in Colombia. These findings underscore the need for a tomato seed certification program in Colombia to ensure viral-free seeds.
Keywords
Diagnosis, Plant virology, RT-qPCR, Sequencing, Solanaceae
References
- Alcalá, R., S. Coşkan, M. Londoño, and J. Polston. 2017. Genome sequence of Southern tomato virus in asymptomatic tomato “Sweet Hearts”. Genome Announc. 5(7), 1-2. Doi: https://doi.org/10.1128/genomeA.01374-16
- Álvarez-Yepes, D., P. Gutiérrez-Sánchez, and M. Marín-Montoya. 2017. Secuenciación del genoma del Potato yellow vein virus (PYVV) y desarrollo de una prueba molecular para su detección. Bioagro 29(1), 3-14.
- Chaves, G., K. Cubillos, and M. Guzmán-Barney. 2014. First report of recombination in Potato yellow vein virus (PYVV) in Colombia. Trop. Plant Pathol. 39(3), 234-241. Doi: https://doi.org/10.1590/S1982-56762014000300007
- Edgar, R.C., R.M. Drive, and M. Valley. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32(5), 1792-1797. Doi: https://doi.org/10.1093/nar/gkh340
- Elvira-González, L., C. Carpino, A. Alfaro-Fernández, M.I. Font-San Ambrosio, R. Peiró, L. Rubio, and L. Galipienso. 2018. A sensitive real-time RT-PCR reveals a high incidence of Southern tomato virus (STV) in Spanish tomato crops. Span. J. Agric. Res. 16(3), e1008. Doi: https://doi.org/10.5424/sjar/2018163-12961
- Elvira-González, L., V. Medina, L. Rubio, and L. Galipienso. 2020. The persistent southern tomato virus modifies miRNA expression without inducing symptoms and cell ultra-structural changes. Eur. J. Plant Pathol. 156(2), 615-622. Doi: https://doi.org/10.1007/s10658-019-01911-y
- Elvira-González, L., A.V. Puchades, C. Carpino, A. Alfaro-Fernández, M.I. Font-San-Ambrosio, L. Rubio, and L. Galipienso. 2017. Fast detection of Southern tomato virus by one-step transcription loop-mediated isothermal amplification (RT-LAMP). J. Virol. Methods 241(3), 11-14. Doi: https://doi.org/10.1016/j.jviromet.2016.12.004
- FAO. 2022. FAOSTAT Crops and livestock products - Tomato. In: https://www.fao.org/faostat/en/; consulted: June, 2024.
- Gallo, Y., P.A. Gutiérrez, and M. Marín, M, 2020a. Identificación de virus de RNA en cultivos de tomate del oriente de Antioquia (Colombia) por secuenciación de nueva generación (NGS). Rev. U.D.C.A Act. & Div. Cient. 23(1), e1414. Doi: http://doi.org/10.31910/rudca.v23.n1.2020.1414
- Gallo, Y., L. Muñoz, L. Toro, P.A. Gutiérrez, and M. Marín. 2020b. Detección de virus de ARN en cultivos de tomate del Oriente Antioqueño mediante secuenciación de alto rendimiento y RT-qPCR. Bioagro 32(3), 147-158.
- García, A., S. Giraldo, M. Higuita, R. Hoyos, M. Marín, and P. Gutiérrez. 2023. Detection of RNA viruses in potato seed-tubers from northern Antioquia (Colombia). Rev. Ceres 70(5), 1-10. Doi: https://doi.org/10.1590/0034-737x202370050013
- Gil, J.F., J.M. Cotes, and M. Marín. 2013. Incidencia visual de síntomas asociados a enfermedades virales en cultivos de papa de Colombia. Rev. Bio. Agron. 11(2), 101-110.
- Green, K.J., C.N. Funke, J. Chojnacky, R.A. Alvarez-Quinto, J.B. Ochoa, D.F. Quito-Avila, and A.V. Karasev. 2020. Potato virus Y (PVY) isolates from Solanum betaceum represent three novel recombinants within the PVYN strain group and are unable to systemically spread in potato. Phytopathology 110(9), 1588-1596. Doi: https://doi.org/10.1094/PHYTO-04-20-0111-R
- Guzmán, M., L. Franco-Lara, D. Rodríguez, L. Vargas, and J.E. Fierro. 2012. Yield losses in Solanum tuberosum Group Phureja cultivar Criolla Colombia in plants with symptoms of PYVV in field trials. Am. J. Potato Res. 89(6), 438-447. Doi: https://doi.org/10.1007/s12230-012-9265-0
- Hao, X., Y. Zheng, B. Cui, and B. Xiang. 2023. Localization of southern tomato virus (STV) in tomato tissues. J. Plant Dis. Prot. 130, 1143-1147. Doi: https://doi.org/10.1007/s41348-023-00729-5
- Henao-Díaz, E., P. Gutiérrez-Sánchez, and M. Marín-Montoya. 2013. Análisis filogenético de aislamientos del Potato virus Y (PVY) obtenidos en cultivos de papa (Solanum tuberosum) y tomate de árbol (Solanum betaceum) en Colombia. Actual. Biol. 35(99), 219-232. Doi: https://doi.org/10.17533/udea.acbi.329120
- Hernández-Guzmán, A.K. and M. Guzmán-Barney. 2014. Detección del virus del amarillamiento de las nervaduras de la hoja de la papa en diferentes órganos de Solanum tuberosum grupo Phureja cv Criolla Colombia utilizando RT-PCR convencional y en tiempo real. Rev. Colomb. Biotecnol. 16(1), 74-85. Doi: https://doi.org/10.15446/rev.colomb.biote.v16n1.44226
- Karasev, A.V. and S.M. Gray. 2013. Continuous and emerging challenges of Potato virus Y in potato. Annu. Rev. Phytopathol. 51, 571-586. Doi: https://doi.org/10.1146/annurev-phyto-082712-102332
- Livieratos, I., E. Eliasco, G. Muller, R. Olsthoorn, L. Salazar, W. Pleij, and R.H.A. Coutts. 2004. Analysis of the RNA of Potato yellow vein virus: evidence for a tripartite genome and conserved 3’-terminal structures among members of the genus Crinivirus. J. Gen. Virol. 85(7), 2065-2075. Doi: https://doi.org/10.1099/vir.0.79910-0
- López, R., C. Asensio, M. Gúzman, and N. Boonham. 2006. Development of real-time and conventional RT-PCR assays for the detection of Potato yellow vein virus (PYVV). J. Virol. Methods 136(1-2), 24-29. Doi: https://doi.org/10.1016/j.jviromet.2006.03.026
- Marín, M. and P. Gutiérrez. 2016. Principios de virología molecular de plantas tropicales. Corpoica, Bogota. Doi: https://doi.org/10.21930/978-958-740-229-2
- MinAgricultura, Ministerio de Agricultura y Desarrollo Rural Colombia. 2021. Agrone: área, producción y rendimiento nacional por cultivo - tomate. In: www.agronet.gov.co/estadistica/Paginas/home.aspx; consulted: June, 2024.
- Morales, F., P. Tamayo, M. Castaño, C. Olaya, A. Martínez, and A. Velasco. 2009. Enfermedades virales del tomate (Solanum lycopersicum) en Colombia. Fitopatol. Colomb. 33(1), 23-27.
- Muñoz, L., P. Gutiérrez-Sánchez, and M. Marín-Montoya. 2016. Detección y secuenciación del genoma del Potato Virus Y (PVY) que infecta plantas de tomate en Antioquia, Colombia. Bioagro 28(2), 69-80.
- Muñoz, L., P. Gutiérrez-Sánchez, and M. Marín-Montoya. 2017. Secuenciación del genoma completo del Potato yellow vein virus (PYVV) en tomate (Solanum lycopersicum) en Colombia. Acta Biol. Colomb. 22(1), 5-17. Doi: https://doi.org/10.15446/abc.v22n1.59211
- Nie, X. and R.P. Singh. 2001. A novel usage of random primers for multiplex RT-PCR detection of virus and viroid in aphids, leaves and tubers. J. Virol. Methods 91(1), 37-49. Doi: https://doi.org/10.1016/S0166-0934(00)00242-1
- Offei, S.K., N. Arciniegas, G. Muller, M. Guzmán, L.F. Salazar, and R.H. Coutts. 2004. Molecular variation of Potato yellow vein virus isolates. Arch. Virol. 49(4), 821-827. Doi: https://doi.org/10.1007/s00705-003-0250-2
- Ogawa, T., Y. Tomitaka, A. Nakagawa, and K. Ohshima. 2008. Genetic structure of a population of Potato virus Y inducing potato tuber necrotic ringspot disease in Japan; comparison with North American and European populations. Virus Res. 131(2), 199-212. Doi: https://doi.org/10.1016/j.virusres.2007.09.010
- Pagán, I. 2022. Transmission through seeds: The unknown life of plant viruses. PLoS Pathog. 18(8), e1010707. Doi: https://doi.org/10.1371/journal.ppat.1010707
- Petrov, N. 2014. Damaging effects of tomato mosaic virus and Potato virus Y on tomato plants. Sci. Technol. 4(6), 56-60.
- Prigigallo, M.I., M. Križnik, D. De Paola, D. Catalano, K. Gruden, M.M. Finetti-Sialer, and F. Cillo. 2019. Potato virus Y infection alters small RNA metabolism and immune response in tomato. Viruses 11(12), e1100. Doi: https://doi.org/10.3390/v11121100
- Rívarez, M.P.S., A. Pecman, K. Bačnik, O. Maksimović, A. Vučurović, G. Seljak, N. Mehle, I. Gutiérrez-Aguirre, M. Ravnikar, and D. Kutnjak. 2023. In-depth study of tomato and weed viromes reveals undiscovered plant virus diversity in an agroecosystem. Microbiome 11(1), 60. Doi: https://doi.org/10.1186/s40168-023-01500-6
- Rívarez, M.P.S., A. Vučurović, N. Mehle, M. Ravnikar, and D. Kutnjak. 2021. Global advances in tomato virome research: current status and the impact of high-throughput sequencing. Front Microbiol. 12, 671925. Doi: https://doi.org/10.3389/fmicb.2021.671925
- Rodríguez, M.H., N.E. Niño, J. Cutler, J. Langer, F. Casierra-Posada, D. Miranda, M. Bandte, and C. Büttner. 2016. Certificación de material vegetal sano en Colombia: un análisis crítico de oportunidades y retos para controlar enfermedades ocasionadas por virus. Rev. Colomb. Cienc. Hortic. 10(1), 164-175. Doi: http://dx.doi.org/10.17584/rcch.2016v10i1.4921
- Sabanadzovic, S., R.A.Valverde, J.K. Brown, R.R. Martin, and I.E. Tzanetakis. 2009. Southern tomato virus: The link between the families Totiviridae and Partitiviridae. Virus Res. 140(1-2), 130-137. Doi: https://doi.org/10.1016/j.virusres.2008.11.018
- Salazar, L., G. Muller, M. Querci, J. Zapata, and R. Owens. 2000. Potato yellow vein virus: its host range, distribution in South America and identification as a Crinivirus transmitted by Trialeurodes vaporariorum. Ann. Appl. Biol. 137(1), 7-19. Doi: https://doi.org/10.1111/j.1744-7348.2000.tb00052.x
- Singh, M., R.P. Singh, and M.S. Fageria. 2012. Optimization of a Real-Time RT-PCR assay and its comparison with ELISA, conventional RT-PCR and the grow-out test for large scale diagnosis of potato virus Y in dormant potato tubers. Am. J. Potato Res. 90(1), 43-50. Doi: https://doi.org/10.1007/s12230-012-9274-z
- Tamayo, P. and P. Jaramillo. 2013. Enfermedades del tomate, pimentón, ají y berenjena en Colombia. Guía para su diagnóstico y manejo. Corpoica, Rionegro, Colombia. Doi: https://doi.org/10.21930/978-958-740-166-0
- Tamura, K., G. Stecher, and S. Kumar. 2021. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 38(7), 3022-3027. Doi: https://doi.org/10.1093/molbev/msab120
- Tsedaley, B. 2015. A review paper on Potato virus Y (PVY) biology, economic importance and its managements. J. Biol. Agric. Healthc. 5(9), 110-127.
- Vaca-Vaca, J.C., J.F. Betancur-Pérez, and K. López-López. 2012. Distribución y diversidad genética de Begomovirus que infectan tomate (Solanum lycopersicum L.) en Colombia. Rev. Colomb. Biotecnol. 14(1), 60-76.
- Villamil-Garzón, A., W.J. Cuellar, and M. Guzmán-Barney. 2014. Natural co-infection of Solanum tuberosum crops by the Potato yellow vein virus and potyvirus in Colombia. Agron. Colomb. 32(2), 213-223. Doi: https://doi.org/10.15446/agron.colomb.v32n2.43968
