In Vitro antifungal activity of ethanol extracts from Cnidoscolus urens L. in controlling Colletotrichum spp. in Lycopersicum esculentum: a sustainable agricultural perspective
Abstract
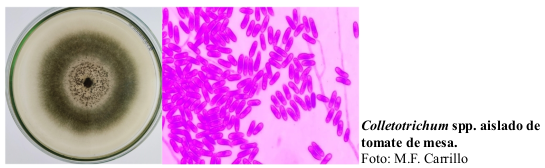
Anthracnose, caused by the pathogenic fungi Colletotrichum spp., poses a significant threat to table tomato (Lycopersicum esculentum) cultivation. This study delves into the potential of plant extracts from Cnidoscolus urens L. as an alternative biocontrol strategy to combat this disease. Rich in secondary metabolites like terpenes, which are instrumental in plant defense, these extracts also comprise esters and fatty acids. Although the latter are not classified as secondary metabolites, they contribute significantly to the plant's biochemical makeup. Our objective was to gauge the in vitro inhibitory efficacy of ethanolic extracts derived from the leaves and stems of Cnidoscolus urens L. against Colletotrichum spp. To achieve this, an agar dilution method with varying extract concentrations was employed. The results showed that concentrations ranging from treatment 3 to treatment 8 effectively inhibited fungal mycelial growth. Interestingly, the extracts' origin, whether from leaves or stems, did not show any significant differential impact on their inhibitory activity. These insights emphasize the consistent effect of Cnidoscolus urens L. extracts in stalling Colletotrichum spp. growth, underscoring their potential as biological antifungal agents in agriculture. Given the pronounced in vitro effectiveness of both leaf and stem extracts, they beckon further exploration as part of sustainable agricultural strategies to combat prominent diseases like anthracnose.
Keywords
Agar dilution method, Secondary metabolites, In Vitro inhibition, Anthracnose biocontrol
References
- Alarcon, J., D.C. Recharte, F. Yanqui, S.M. Moreno, and M.A. Buendía. 2020. Fertilizar con microorganismos eficientes autóctonos tiene efecto positivo en la fenología, biomasa y producción de tomate (Lycopersicum esculentum Mill). Sci. Agropecu. 11(1), 67-73. Doi: https://doi.org/10.17268/sci.agropecu.2020.01.08
- Álvarez, E., C.A. Ospina, J.F. Mejía de los Ríos, and G.A. Llano. 2004. Caracterización morfológica, patogénica y genética del agente causal de la antracnosis (Colletotrichum gloeosporioides) en guanábana (Annona muricata) en el Valle del Cauca. Fitopatol. Colomb. 28(1), 1-8. Doi: https://cgspace.cgiar.org/handle/10568/44257
- Barnett, H.L., and Hunter, B. B. 1986. Illustrated genera of imperfect fungi. 4th ed. Macmillan Publishing Co., New York, NY.
- Benson, D.A., I, Karsch., D.J. Lipman, J. Ostell, and D.L. Wheeler. 2005. GenBank. NAR. 1(33), 34-38. Doi: https://doi.org/10.1093/nar/gki063
- Carvalho Neto, M.F., R.C.R.G. Gervásio, E.C.C. Araújo, J.C. Almeida, and A.P. Oliveira. 2018. Bioactivity of the organic extracts of Cnidoscolus urens (L.) Arthur (Euphorbiaceae) on the cabbage-caterpillar. Comun. Sci. 9(3), 402-411. Doi: https://doi.org/10.14295/cs.v8i1.2556
- Chaves-Bedoya, G. 2022. Study on Croton sp. genetic diversity in the department of Norte de Santander using the internal transcribed spacer (ITS) region of ribosomal DNA (rDNA). Rev. Colomb. Cienc. Hortic. 16(1), e13592. Doi: https://doi.org/10.17584/rcch.2022v16i1.13592
- Chaves-Bedoya, G. and L. Ortiz-Rojas. 2022. Estudio fitoquímico de Cnidoscolus urens (L.) Arthur procedente de la región de Cúcuta (Colombia). Inf. Tecnol. 33(6), 21-30. Doi: https://doi.org/10.4067/S0718-07642022000600021
- Ciofini, A., F. Negrini, R. Baroncelli, and E. Baraldi. 2022. Management of post-harvest anthracnose: current approaches and future perspectives. Plants 11(14), 1856, Doi: https://doi.org/10.3390/plants11141856
- El Khetabi, A., R. Lahlali, S. Ezrari, N. Radouane, N. Lyousfi, H. Banani, L. Askarne, A. Tahiri, L. El Ghadraoui, S. Belmalha, and E.A. Barka. 2022. Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: a review. Trends Food Sci. Tech. 120, 402-417. Doi: https://doi.org/10.1016/j.tifs.2022.01.009
- Elhamouly, N.A., O.A. Hewedy, A. Zaitoon, A. Miraples, O.T. Elshorbagy, S. Hussien, A. El-Tahan, and D. Peng. 2022. The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation. Front. Plant Sci. 13, 1044896. Doi: https://doi.org/10.3389/fpls.2022.1044896
- Figueiredo Junior, E.C., Y.W. Cavalcanti, A.B. Lira, H.L.F. Pessoa, W.S. Lopes, D.R. Silva, I.A. Freires, P.L. Rosalen, E.M.M.B. Costa, and J.V. Pereira. 2021. Phytochemical composition, antifungal activity, in vitro and in vivo toxicity of Syzygium cumini (L.) skeels leaves extract. Bol. Latinoam. Caribe Plantas Med. Aromat. 20(5), 536-557. Doi: https://doi.org/10.37360/blacpma.21.20.5.40
- Kong, W.L., L. Rui, H. Ni, and X.Q. Wu. 2020. Antifungal effects of volatile organic compounds produced by Rahnella aquatilis JZ-GX1 against Colletotrichum gloeosporioides in Liriodendron chinense × tulipifera. Front. Microbiol. 11, 1114. Doi: https://doi.org/10.3389/fmicb.2020.01114
- Martínez-Culebras, P.V. 1999. Caracterización y diagnóstico molecular de las cepas de Colletotrichum patógenas de plantas de fresa. PhD thesis. Universidad de Valencia, Valencia, Spain.
- Panno, S., S. Davino, A.G. Caruso, S. Bertacca, A. Crnogorac, A. Mandić, E. Noris, and S. Matić. 2021. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the Mediterranean basin. Agronomy 11(11), 2188. Doi: https://doi.org/10.3390/agronomy11112188
- Percival, G.C. and S. Graham. 2021. Evaluation of inducing agents and synthetic fungicide combinations for management of foliar pathogens of urban trees. Arboric. Urban For. 47(2), 85-95. Doi: https://doi.org/10.48044/jauf.2021.008
- Quiroz-Lobo, Y., G. Santafé-Patiño, and J.-A. Quirós-Rodríguez. 2022. Bioactividad e identificación de los ácidos grasos de la esponja marina Tetilla rodriguesi (Tetractinellida: Tetillidae) en el Caribe colombiano. Rev. Biol. Trop. 70(1), 20-29.
- Sepúlveda-Flórez, D.R. 2016. Sistemas de producción de tomate en el municipio de Cáchira, Norte de Santander: en busca de elementos para el análisis de su sostenibilidad. Undergraduate thesis. Pontificia Universidad Javeriana, Bogota.
- Shahriar, S.A., A. Husna, T.T. Paul, M.N.K. Eaty, M. Quamruzzaman, A.B. Siddique, M.A. Rahim, A.N.F. Ahmmed, J. Uddain, and S. Siddiquee. 2023. Colletotrichum truncatum causing anthracnose of tomato (Solanum lycopersicum L.) in Malaysia. Microorganisms 11(1), 226. Doi: https://doi.org/10.3390/microorganisms11010226
- Vaou, N., E. Stavropoulou, C. Voidarou, Z. Tsakris, G. Rozos, C. Tsigalou, and E. Bezirtzoglou. 2022. Interactions between medical plant-derived bioactive compounds: focus on antimicrobial combination effects. Antibiotics 11(8), 1014. Doi: https://doi.org/10.3390/antibiotics11081014
- Waheed, K., H. Nawaz, M.A. Hanif, and R. Rehman. 2020. Tomato. pp. 631-644. In: Hanif, M.A., H. Nawaz, M.M. Khan, and H.J. Byrne (eds.). Medicinal plants of South Asia: novel sources for drug discovery. Elsevier, Doi: https://doi.org/10.1016/B978-0-08-102659-5.00046-X
- Yuan, H., Q. Ma, L. Ye, and G. Piao. 2016. The traditional medicine and modern medicine from natural products. Molecules 21(5), 559. Doi: https://doi.org/10.3390/molecules21050559
