Sampling methods as a basis for assessing the phytosanitary status of diseases and damage in blueberry production in the Colombian highland tropics
Abstract
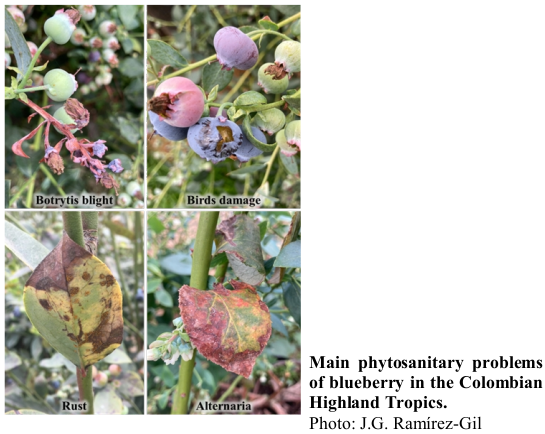
Blueberry production has significantly increased globally in recent years due to its excellent nutritional quality. In Colombia, cultivated areas have expanded, along with the rise of phytosanitary problems that are still not well-characterized, nor are there reliable sampling tools for monitoring and evidence-based decision-making. This study focuses on the symptomatological characterization, identification, and determination of the incidence of the main blueberry pathologies (diseases and disorders) in Colombia, under high-altitude tropical conditions. Additionally, various sampling methods were evaluated as a basis for developing statistically valid tools for monitoring in field conditions. The research was conducted in commercial fields across nine municipalities in the Cundinamarca and Boyaca regions of Colombia. After characterizing the main diseases and disorders, the incidence was determined under field conditions, and the best sampling strategy was evaluated based on methods such as random, systematic grid-based, and stratified sampling. Sample size determination was based on the finite population method. The intensity measures evaluated incidence and severity showed that in Colombia’s high-altitude tropics, the most important diseases were rust and shoot dieback, mechanical damage, and fruit dehydration as abiotic disorders. Stratified sampling yielded the best performance, showing the lowest coefficient of variation. Our findings provide the first characterization of blueberry pathologies, their significance, and a sampling method for evidence-based decision-making. This work is crucial as it establishes a methodology for identifying phytosanitary issues, proposes a robust sampling approach for field application, and emphasizes the need for ongoing detection of emerging diseases to enhance data-driven decision-making.
Keywords
Vaccinium corymbosum, Stratified sampling, Dieback, Rust, Incidence, Severity
References
- Abbey, J., D. Percival, L. Jaakola, and S.K. Asiedu. 2024. Efficacy, persistence and residue levels of fungicides for Botrytis control in wild blueberry. Crop Prot. 179, 106633. Doi: https://doi.org/10.1016/j.cropro.2024.106633
- Appelhoff, S., R. Hertwig, and B. Spitzer. 2022. Control over sampling boosts numerical evidence processing in human decisions from experience. Cereb. Cortex 33(1), 207. Doi: https://doi.org/10.1093/cercor/bhac062
- Aubertot, J.N., J.J. Schott, A. Penaud, H. Brun, and T. Doré. 2004. Methods for sampling and assessment in relation to the spatial pattern of phoma stem canker (Leptosphaeria maculans) in oilseed rape. Eur. J. Plant Pathol. 110(2), 183-192. Doi: https://doi.org/10.1023/B:EJPP.0000015359.61910.3b
- Banerjee, S., G.A. Nayik, J. Kour, and N. Nazir. 2020. Blueberries. pp. 593-614. In: Nayik G.A. and A. Gull (eds.). Antioxidants in fruits: properties and health benefits. Springer, Singapore. Doi: https://doi.org/10.1007/978-981-15-7285-2_31
- Belan, L.L., L.L. Belan, L.S. Satiro, W.B. Moraes, W.C. de Jesus Junior, and W.B. Moraes. 2021. A comparison of sampling methods to accurately estimate the incidence of leaf rust on conilon coffee. Australas. Plant Pathol. 50(6), 729-737. Doi: https://doi.org/10.1007/s13313-021-00823-y
- Binns, M.R. and J.P. Nyrop. 1992. Sampling insect populations for the purpose of IPM decision making. Ann. Rev. Entomol. 37, 427-453. Doi: https://doi.org/10.1146/annurev.en.37.010192.002235
- Binns, M.R., J.P. Nyrop, and W. van der Werf. 2000. Sampling and monitoring in crop protection: The theoretical basis for developing practical decision guides. CABI, Wallingford, UK.
- Bock, C.H., K.S. Chiang, and E.M. Del Ponte. 2022. Plant disease severity estimated visually: a century of research, best practices, and opportunities for improving methods and practices to maximize accuracy. Trop. Plant Pathol. 47(1), 25-42. Doi: https://doi.org/10.1007/s40858-021-00439-z
- Brown, C.E. 1998. Coefficient of variation. pp. 155-157. In: Brown, C.E. (ed.). Applied multivariate statistics in geohydrology and related sciences. Springer, Berlin. Doi: https://doi.org/10.1007/978-3-642-80328-4_13
- Cleves, J.A. 2021. Fundamentos técnicos del cultivo del arándano (Vaccinium corymbosum L.) en la región central de Colombia. Editorial UPTC, Tunja, Colombia. Doi: https://doi.org/10.19053/9789586604895
- Cline, W.O. and H.J. Burrack. 2023. Diseases and insects of economic importance affecting blueberry fruit in North Carolina. Acta Hortic. 1357, 229-234. Doi: https://doi.org/10.17660/ActaHortic.2023.1357.33
- Cochran, W.G. 1997. Sampling techniques. 3rd ed. John Wiley and Sons, New York, NY.
- de Silva, A., K. Patterson, C. Rothrock, and R. McNew. 1999. Phytophthora root rot of blueberry increases with frequency of flooding. HortScience 34(4), 693-695. Doi: https://doi.org/10.21273/HORTSCI.34.4.693
- del Águila, I.M., J. Cañadas, and S. Túnez. 2015. Decision making models embedded into a web-based tool for assessing pest infestation risk. Biosyst. Eng. 133, 102-115. Doi: https://doi.org/10.1016/j.biosystemseng.2015.03.006
- Donoso, A. and S. Valenzuela. 2018. In-field molecular diagnosis of plant pathogens: recent trends and future perspectives. Plant Pathol. 67(7), 1451-1461. Doi: https://doi.org/10.1111/ppa.12859
- Fan, S., C. Li, W. Huang, and L. Chen. 2017. Detection of blueberry internal bruising over time using NIR hyperspectral reflectance imaging with optimum wavelengths. Postharvest Biol. Technol. 134, 55-66. Doi: https://doi.org/10.1016/j.postharvbio.2017.08.012
- Fischer, G., D. Miranda, S. Magnitskiy, H.E. Balaguera-López, and Z. Molano (eds.). 2021. Avances en el cultivo de las berries en el trópico. SCCH, Bogota. Doi: https://doi.org/10.17584/IBerries
- García-León, E., J.M. Tovar-Pedraza, L.A. Valbuena-Gaona, V.H. Aguilar-Pérez, K.Y. Leyva-Madrigal, G.A. Mora-Romero, and J. G. Ramírez-Gil. 2024. Identification of the causal agent of Guar leaf blight and development of a semi-automated method to quantify disease severity. Trop. Plant Pathol. 49, 825-837. Doi: https://doi.org/10.1007/s40858-024-00676-y
- Hariharan, G. and K. Prasannath. 2021. Recent advances in molecular diagnostics of fungal plant pathogens: a mini review. Front. Cell. Infect. Microbiol. 10, 600234. Doi: https://doi.org/10.3389/fcimb.2020.600234
- Hilário, S., A. Lopes, L. Santos, and A. Alves. 2020. Botryosphaeriaceae species associated with blueberry stem blight and dieback in the Centre Region of Portugal. Eur. J. Plant Pathol. 156(1), 31-44. Doi: https://doi.org/10.1007/s10658-019-01860-6
- Holland, R.M., R.S.C. Christiano, E. Gamliel-Atinsky, and H. Scherm. 2014. Distribution of Xylella fastidiosa in blueberry stem and root sections in relation to disease severity in the field. Plant Dis. 98(4), 443-447. Doi: https://doi.org/10.1094/PDIS-06-13-0680-RE
- Hou, J., B. Park, C. Li, and X. Wang. 2024. A multiscale computation study on bruise susceptibility of blueberries from mechanical impact. Postharvest Biol. Technol. 208, 112660. Doi: https://doi.org/10.1016/j.postharvbio.2023.112660
- Hughes, G. 1999. Sampling for decision making in crop loss assessment and pest management: introduction. Phytopathology 89(11), 1080-1083. Doi: https://doi.org/10.1094/PHYTO.1999.89.11.1080
- Lagos-Ortiz, K., J. Medina-Moreira, A. Alarcón-Salvatierra, M.F. Morán, J. del Cioppo-Morstadt, and R. Valencia-García. 2019. Decision support system for the control and monitoring of crops. pp. 20-28. In: Valencia-García, R., G. Alcaraz-Mármol, J. del Cioppo-Morstadt, N. Vera-Lucio, and M. Bucaram-Leverone (eds.). ICT for Agriculture and Environment. Springer, Cham, Switzerland. Doi: https://doi.org/10.1007/978-3-030-10728-4_3
- Lin, C.S., G. Poushinsky, and M. Mauer. 1979. An examination of five sampling methods under random and clustered disease distributions using simulation. Can. J. Plant Sci. 59(1), 121-130. Doi: https://doi.org/10.4141/cjps79-017
- Liu, S., W. Liu, X. Zhou, and P. Gong. 2012. Kernel-based parametric analytical model of source intensity distributions in lithographic tools. Appl. Opt. 51(10), 1479-1486. Doi: https://doi.org/10.1364/AO.51.001479
- Lucas, J.A. 2011. Advances in plant disease and pest management. J. Agric. Sci. 149(1), 91-114. Doi: https://doi.org/10.1017/S0021859610000997
- Luo, W., S. Pietravalle, S. Parnell, F. van den Bosch, T.R. Gottwald, M.S. Irey, and S.R. Parker. 2012. An improved regulatory sampling method for mapping and representing plant disease from a limited number of samples. Epidemics 4(2), 68-77. Doi: https://doi.org/10.1016/j.epidem.2012.02.001
- Macha, R., F.C. Navarro Soto, A. Ramírez Ríos, and E.A. Alfaro Paredes. 2023. International market concentration of fresh blueberries in the period 2001-2020. Hum. Soc. Sci. Commun. 10(1), 1-12. Doi: https://doi.org/10.1057/s41599-023-02455-7
- Madden, L.V. and G. Hughes. 1999a. An effective sample size for predicting plant disease incidence in a spatial hierarchy. Phytopathology 89(9), 770-781. Doi: https://doi.org/10.1094/PHYTO.1999.89.9.770
- Madden, L.V. and G. Hughes. 1999b. Sampling for plant disease incidence. Phytopathology 89(11), 1088-1103. Doi: https://doi.org/10.1094/PHYTO.1999.89.11.1088
- Madden, L.V., G. Hughes, and G.P. Munkvold. 1996. Plant disease incidence: inverse sampling, sequential sampling, and confidence intervals when observed mean incidence is zero. Crop Prot. 15, 621-632. Doi: https://doi.org/10.1016/0261-2194(96)00025-7
- Madden, L.V., G. Hughes, and F. van den Bosch. 2007. The study of plant disease epidemics. APS Press, St. Paul, MN.
- Madrid, M. and R. Beaudry. 2020. Small fruits: raspberries, blackberries, blueberries. pp. 335-346. In: Gil, M.I. and R. Beaudry (eds.), Controlled and modified atmospheres for fresh and fresh-cut produce. Academic Press, London. Doi: https://doi.org/10.1016/B978-0-12-804599-2.00020-X
- Mahaman, B.D., H.C. Passam, A.B. Sideridis, and C.P. Yialouris. 2003. DIARES-IPM: a diagnostic advisory rule-based expert system for integrated pest management in Solanaceous crop systems. Agric. Syst. 76(3), 1119-1135. Doi: https://doi.org/10.1016/S0308-521X(02)00187-7
- Moggia, C., J. Graell, I. Lara, G. González, and G. A. Lobos. 2017. Firmness at harvest impacts postharvest fruit softening and internal browning development in mechanically damaged and non-damaged highbush blueberries (Vaccinium corymbosum L.). Front. Plant Sci. 8, 535. Doi: https://doi.org/10.3389/fpls.2017.00535
- Mohammad-Razdari, A., D. Rousseau, A. Bakhshipour, S. Taylor, J. Poveda, and H. Kiani. 2022. Recent advances in E-monitoring of plant diseases. Biosens. Bioelectron. 201, 113953. Doi: https://doi.org/10.1016/j.bios.2021.113953
- Nelson, T.A. and B. Boots. 2008. Detecting spatial hot spots in landscape ecology. Ecography 31(5), 556-566. Doi: https://doi.org/10.1111/j.0906-7590.2008.05548.x
- Nutter, F.W. 1993. Assessing the accuracy, intra-rater repeatability, and inter-rater reliability of disease assessment systems. Phytopathology 83(8), 806. Doi: https://doi.org/10.1094/Phyto-83-806
- Nutter, F.W., P.D. Esker, and R.A.C. Netto. 2006. Disease assessment concepts and the advancements made in improving the accuracy and precision of plant disease data. Eur. J. Plant Pathol. 115(1), 95-103. Doi: https://doi.org/10.1007/s10658-005-1230-z
- Pedregosa, F., G. Varoquaux, A. Gramfort, V. Michel, B. Thirion, O. Grisel, M. Blondel, P. Prettenhofer, R. Weiss, V. Dubourg, J. Vanderplas, A. Passos, D. Cournapeau, M. Brucher, M. Perrot, and É. Duchesnay. 2011. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12(85), 2825-2830.
- Plantegenest, M., C. Le May, and F. Fabre. 2007. Landscape epidemiology of plant diseases. J. R. Soc. Interface, 4(16), 963-972. Doi: https://doi.org/10.1098/rsif.2007.1114
- Ramírez-Gil, J.G. and J.G. Morales. 2019. Polyphasic identification of preharvest pathologies and disorders in avocado cv. Hass. Agron. Colomb. 37(3), 213-227. Doi: https://doi.org/10.15446/agron.colomb.v37n3.78528
- Ramírez-Gil, J.G. and J.G. Morales-Osorio. 2018. Microbial dynamics in the soil and presence of the avocado wilt complex in plots cultivated with avocado cv. Hass under ENSO phenomena (El Niño – La Niña). Sci. Hortic. 240, 273-280. Doi: https://doi.org/10.1016/j.scienta.2018.06.047
- Rodriguez-Saona, C., C. Vincent, and R. Isaacs. 2019. Blueberry IPM: past successes and future challenges. Ann. Rev. Entomol. 64, 95-114. Doi: https://doi.org/10.1146/annurev-ento-011118-112147
- Routray, W. and V. Orsat. 2011. Blueberries and their anthocyanins: factors affecting biosynthesis and properties. Compr. Rev. Food Sci. Food Saf. 10(6), 303-320. Doi: https://doi.org/10.1111/j.1541-4337.2011.00164.x
- Ru, S., S. Ding, J.E. Oliver, and A. Amodu. 2023. A review of Botryosphaeria stem blight disease of blueberry from the perspective of plant breeding. Agriculture 13(1), 1. Doi: https://doi.org/10.3390/agriculture13010100
- Sabtu, N.M., N.H. Idris, and M.H.I. Ishak. 2018. The role of geospatial in plant pests and diseases: an overview. IOP Conf. Ser.: Earth Environ. Sci. 169(1), 012013. Doi: https://doi.org/10.1088/1755-1315/169/1/012013
- Savary, S., B. Mille, B. Rolland, and P. Lucas. 2006. Patterns and management of crop multiple pathosystems. Eur. J. Plant Pathol. 115(1), 123-138. Doi: https://doi.org/10.1007/s10658-005-0651-z
- Scherm, H., A.T. Savelle, P.M. Brannen, and G. Krewer. 2008. Occurrence and prevalence of foliar diseases on blueberry in Georgia. Plant Health Prog. 9(1), 18. Doi: https://doi.org/10.1094/PHP-2008-0421-01-RS
- Schillaci, M.A. and M.E. Schillaci. 2022. Estimating the population variance, standard deviation, and coefficient of variation: sample size and accuracy. J. Hum. Evol. 171, 103230. Doi: https://doi.org/10.1016/j.jhevol.2022.103230
- Shechtman, O. 2013. The coefficient of variation as an index of measurement reliability. pp. 39-49. In: Doi, S.A.R. and G.M. Williams (eds.). Methods of clinical epidemiology. Springer, Berlin. Doi: https://doi.org/10.1007/978-3-642-37131-8_4
- Silva, S., E.M. Costa, M. Veiga, R.M. Morais, C. Calhau, and M. Pintado. 2020. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 60(2), 181-200. Doi: https://doi.org/10.1080/10408398.2018.1518895
- Singh, R. and N.S. Mangat. 1996. Stratified sampling. pp. 102-144. In: Singh, R. and N.S. Mangat (eds.). Elements of survey sampling. Springer, Dordrecht, Netherlands. Doi: https://doi.org/10.1007/978-94-017-1404-4_5
- Turechek, W.W. and L.V. Madden. 2001. Effect of scale on plant disease incidence and heterogeneity in a spatial hierarchy. Ecol. Model. 144(1), 77-95. Doi: https://doi.org/10.1016/S0304-3800(01)00350-7
